Tufts Silklab Created Leather-like Material from Silk Proteins
On May 5, 2021, researchers at Tufts University School of Engineering announced they had developed an alternative to…

On May 5, 2021, researchers at Tufts University School of Engineering announced they had developed an alternative to…

On May 5, 2021, the United States sent its first flight loaded with oxygen cylinders and regulators, N95…

On May 5, 2021, the World Health Organization (WHO) and the Federal Republic of Germany announced a new…

On May 5, 2021, Humanigen announced that results from the lenzilumab Phase 3 study in hospitalized COVID-19 patients…

On May 5, 2021, Moderna announced initial data from its Phase 2 study showing that a single 50…

On May 5, 2021, Novavax announced the publication of results from the initial primary analysis of a Phase…

On May 5, 2021, Pfizer and BioNTech announced that Health Canada had expanded the Interim Order authorization for…

On May 5, 2021, Hoth Therapeutics announced it has expanded its sponsored research agreement with Virginia Commonwealth University…

On May 5, 2021, CytoDyn announced an agreement to partner with the Albert Einstein Israelite Hospital (AEIH) in…

On May 4, 2021, HDT Bio announced that its development partner in India, Gennova Biopharmaceuticals, had begun dosing…

On May 4, 2021, Moderna announced an expansion of the Moderna Technology Center (MTC) in Norwood, MA including…

On May 3, 2021, the National Institutes of Health announced that researchers wre able to wirelessly record the…
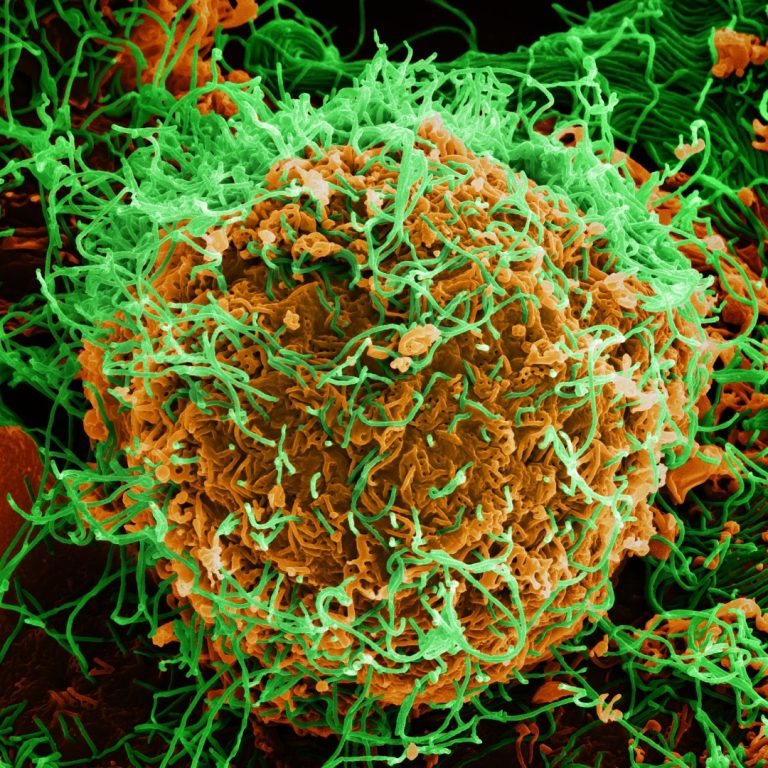
On May 3, 2021, the World Health Organization (WHO) announced the end of the 12th outbreak of Ebola…

On May 3, 2021, the World Health Organization (WHO) welcomed the Government of Swedenメs announcement to share 1…
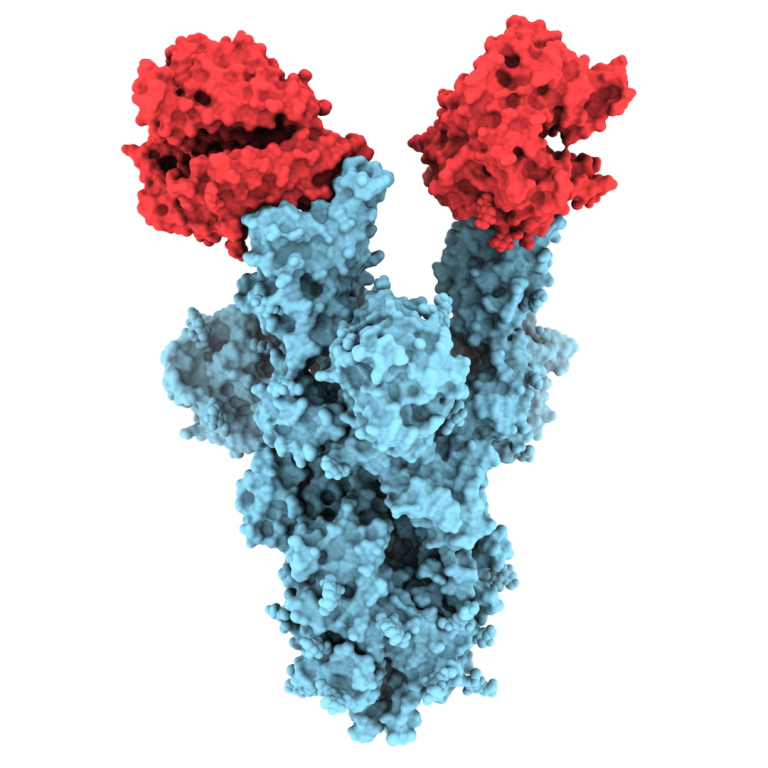
On May 3, 2021, University of British Columbia (UBC) researchers were the first in the world to publish…

On May 3, 2021, Eiger BioPharmaceuticals announced announced that Peginterferon Lambda (Lambda) will be added to the ongoing,…

On May 3, 2021, Vaxart announced that new data obtained from its Phase I COVID-19 trial added to…

On May 3, 2021, Precipio announced that it had successfully launched its COVID-19 rapid antibody test (20 minute)…

On May 3, 2021, Moderna announced an agreement with Gavi, the Vaccine Alliance to supply up to 500…

On May 3, 2021, Novavax announced that it had initiated a pediatric expansion of its Phase 3 clinical…

On May 3, 2021, Gavi, the Vaccine Alliance, that it had signed an advance purchase agreement with Moderna…

On Apr. 30, 2021, the FDA approved AstraZeneca’s Farxiga (dapagliflozin) oral tablets to reduce the risk of kidney…

On Apr. 30, 2021, the FDA revoked the emergency use authorization (EUA) of the Battelle CCDS Critical Care…

On Apr. 30, 2021, Moderna announced that the World Health Organization (WHO) had issued Emergency Use Listing (EUL)…

On Apr. 29, 2021, Lonza announced the expansion of its collaboration with Moderna to manufacture the drug substance…

On Apr. 29, 2021, the National Institutes of Health announced it was funding $29 million in additional grants…
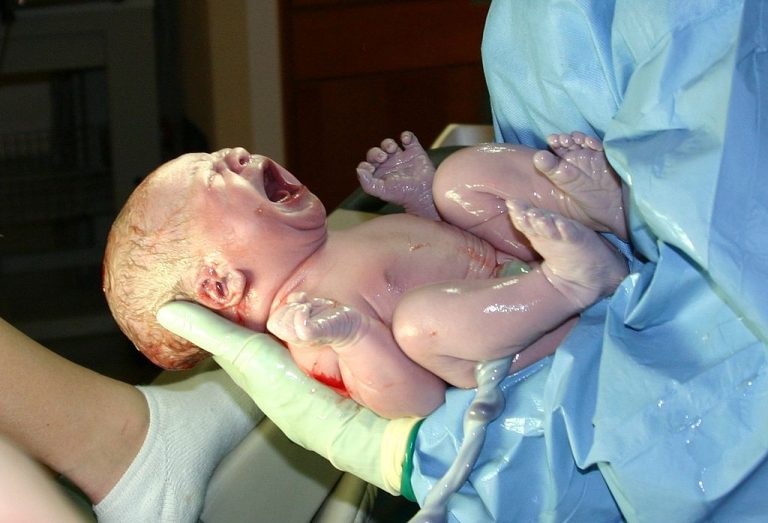
On Apr. 29, 2021, researchers at the University of British Columbia (UBC) announced a study published in Cell…

On Apr. 29, 2021, scientists from several hospitals and research centers reported what happens in individual cells of…

On Apr. 29, 2021, Moderna announced it was making new funding commitments to increase supply at its owned…
On Apr. 29, 2021, Pfizer and BioNTech announced they had submitted a variation to the Conditional Marketing Authorization…