CDC announced H5N1 bird flu virus in U.S. wild birds and poultry posed a low risk to the public
On Feb. 14, 2022, The U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspective Service (APHIS) announced…

On Feb. 14, 2022, The U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspective Service (APHIS) announced…

On Feb. 10, 2022, Vir Biotech announced that preclinical data suggested that sotrovimab, an investigational monoclonal antibody authorized…
On Feb. 10, 2022, Scripps Research announced that the COVID-causing virus SARS-CoV-2 harbors a vulnerable site at the…

On Feb. 4, 2022, the NIH announced a study that showed pregnant women with COVID-19 appeared to be…

On Jan. 27, 2022, Cocrystal Pharma announced that it had selected two investigational novel antiviral drug candidates for further…
On Jan. 27, 2022, NIAID announced that a clinical trial found that the combination of remdesivir plus a…

On Jan. 25. 2022, a report published in PNAS provided strong evidence of extensive SARS-CoV-2 infection of white-tailed…

On Jan. 24, 2022, the University of Oxford announced that a third booster dose of either the ChAdOx1…

On Jan. 24, 2022, Anixa Biosciences announced that the company and its partner, MolGenie, had synthesized a compound…

On Jan. 20, 2022, scientists from the National Institute of Allergy and Infectious Diseases (NIAID) announced that they…

On Jan. 19, 2022, Novavaxa announced that Australia’s Therapeutic Goods Administration had granted approval for provisional registration of…

On Jan. 14, 2022, the U.S. Department of Agriculture’s (USDA) National Veterinary Services Laboratories confirmed a highly pathogenic…
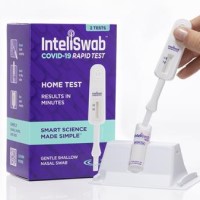
On Jan. 13, 2022, OraSure Technologies that its InteliSwabᆴ COVID-19 rapid tests detected the Omicron variant as effectively…
On Jan. 13, 2022, the Centers for Disease Control and Prevention announced a study published in the journal…
On Jan. 5, 2022, Pfizer and BioNTech announced a new research, development and commercialization collaboration to develop a…

On Jan 5, 2022, Hologic the addition of the Aptimaᆴ SARS-CoV-2 assay to its Global Access Initiative, a…

On Jan 5, 2022, Revelar Biotherapeutics and Twist Bioscience announced that RBT-0813 binded to and neutralized the Omicron…
On Dec. 28, 2021, Pacific Biosciences announced that its new HiFiViral SARS-CoV-2 solution had successfully sequenced and captured…
On Dec. 28, 2021, researchers at the Walter Reed Army Institute of Research in Silver Spring, Maryland announced…
On Dec. 27, 2021, Sorrento Therapeutics announced that initial testing of COVISTIX on recombinant N proteins demonstrated its…

On Dec. 23, 2021, Merck and Ridgeback Biotherapeutics announced that the U.S. Food and Drug Administration (FDA) had…

On Dec. 22, 2021, the University of Oxfordメs vaccine manufacturing research team published a pre-print paper demonstrating the…

On Dec. 22, 2021, Pfizer announced that the U.S. Food and Drug Administration (FDA) had authorized the emergency…
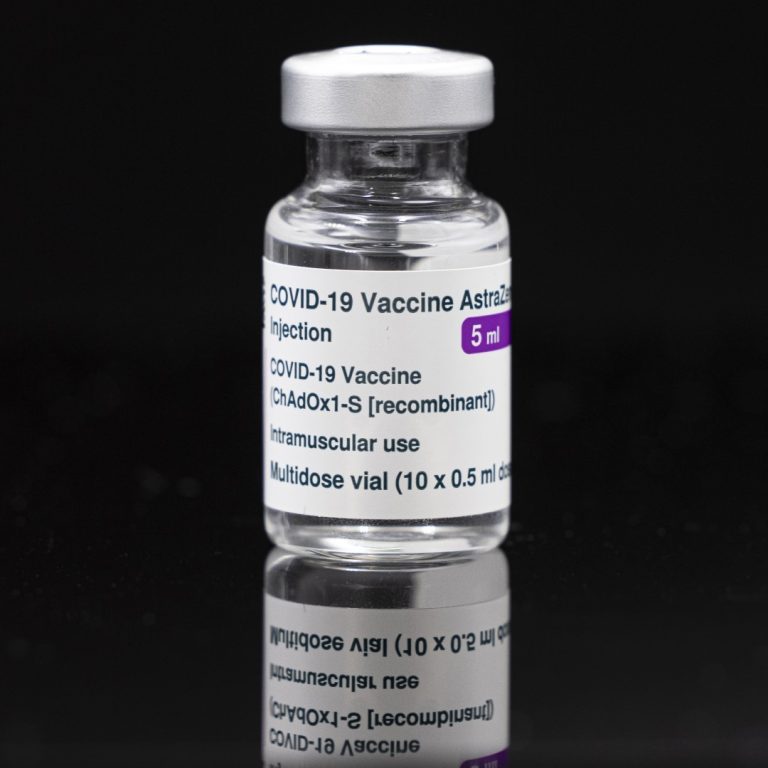
On Dec. 21, 2021, the University of Oxford’s vaccine manufacturing research team published a pre-print paper demonstrating the…

On Dec. 21, 2021, the United States Department of Agriculture’s (USDA) National Veterinary Services Laboratories announced confirmation of…

On Dec. 17, 2021, Novavax and SK bioscience announced that the World Health Organization (WHO) had granted Emergency…

On Dec. 16, 2021, NIH scientists announced that to counter future coronavirus outbreaks, the global scientific and medical…

On Dec. 13, 2021, Moderna announced an agreement with the Australian Government to build a state-of-the-art messenger RNA…

On Dec. 13, 2021, the Coalition for Epidemic Preparedness Innovations, and Affinivax announced a partnership to advance the…

On Dec. 8, 2021, the U.S. Food and Drug Administration issued an emergency use authorization for AstraZenecaメs Evusheld…