The Hybrid Capture II human papillomavirus (HPV) DNA test was approved by the FDA
On Mar. 17, 1999, the Hybrid Capture II human papillomavirus (HPV) DNA test was approved by the U.S….
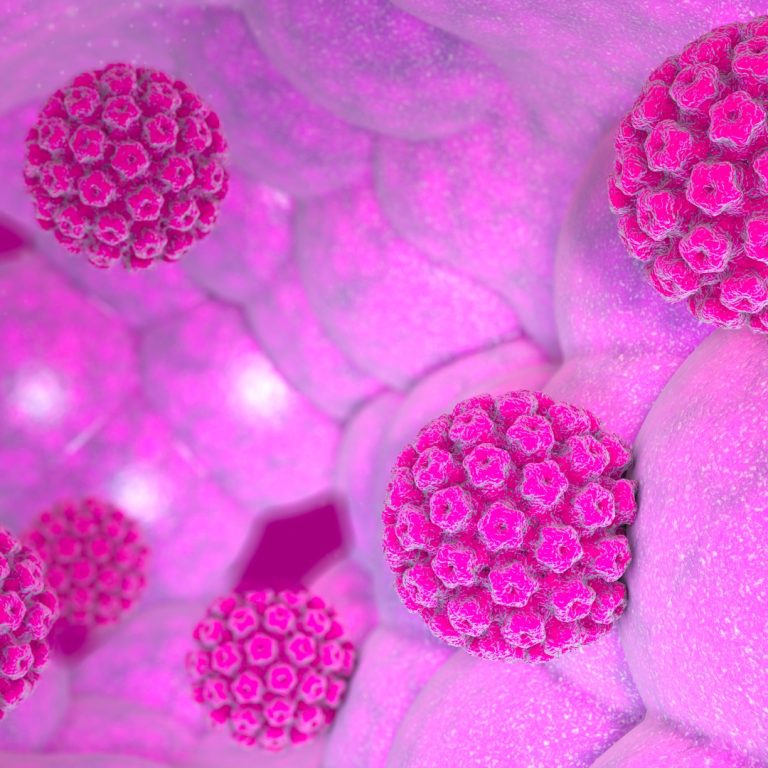
On Mar. 17, 1999, the Hybrid Capture II human papillomavirus (HPV) DNA test was approved by the U.S….

On Aug. 26, 1998, Bill and Melinda Gates announced the establishment of the Bill and Melinda Gates Children’s Vaccine…

In 1998, the U.S. Department of Agriculture (USDA) began surveillance of swine for influenza virus in the U.S….
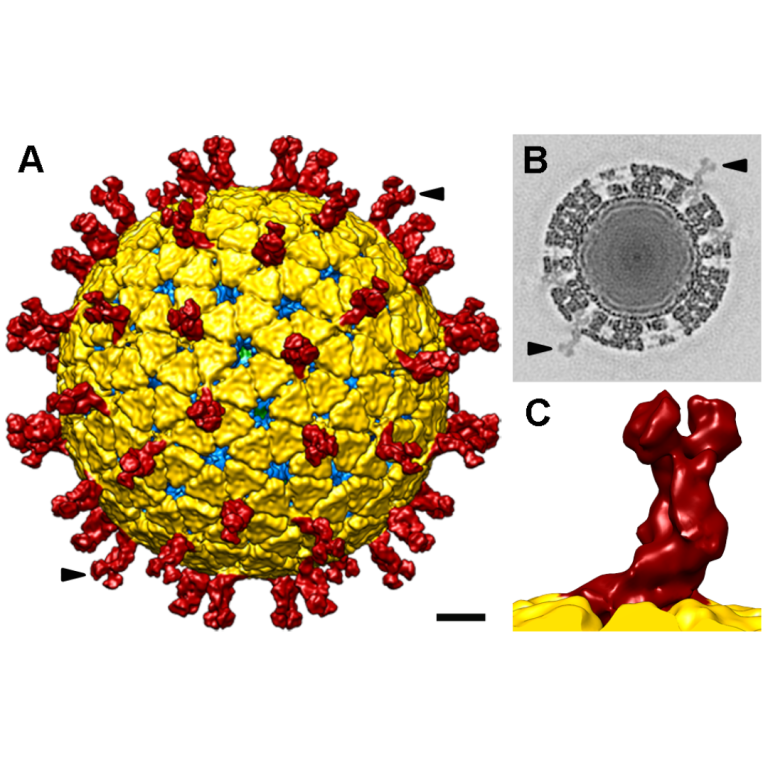
In 1998, University of Calgary’s Dr. Patrick Lee revealed that reovirus injected in mice shrank tumours from brain…
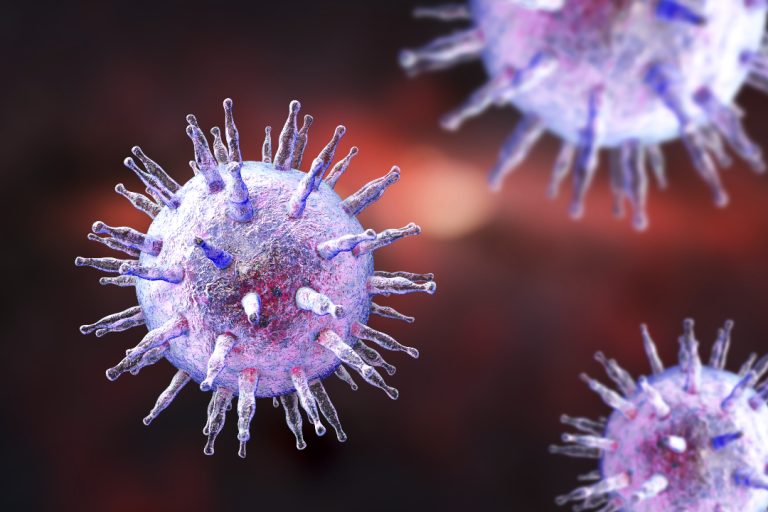
On Dec. 15, 1997, scientists from the Oklahoma Medical Research Foundation (OMRF) announced that Systemic Lupus Erythematosus (lupus)…
In 1997, the first Enterovirus 71 (EV71) epidemic was reported in Malaysia. EV71 is an important emerging pathogen…

In 1997, the Immunization Practices Advisory Committee (ACIP) recommended adoption of a sequential series of two doses of…

In 1997, FluNet, a global web-based tool for influenza virological surveillance was launched. It is a critical tool…

In 1997, the H5N1 bird flu incident in Hong Kong was the first known instance of a purely…

On Jun. 18, 1996, the International AIDS Vaccine Initiative (IAVI) was launched, calling for the speedy development of…

On Mar. 14, 1996, the U.S. Food and Drug Administration (FDA) announced the approval of the first antigen…

In 1996, A/goose/Guangdong/1/1996 (H5N1), the precursor of the highly pathogenic H5N1 avian influenza viruses (HPAIVs) was identified in…
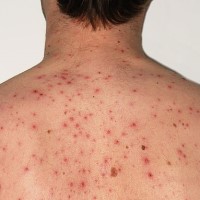
On Mar. 17, 1995, the varicella virus vaccine, live (Varivax by Merck) was licensed for the active immunization…
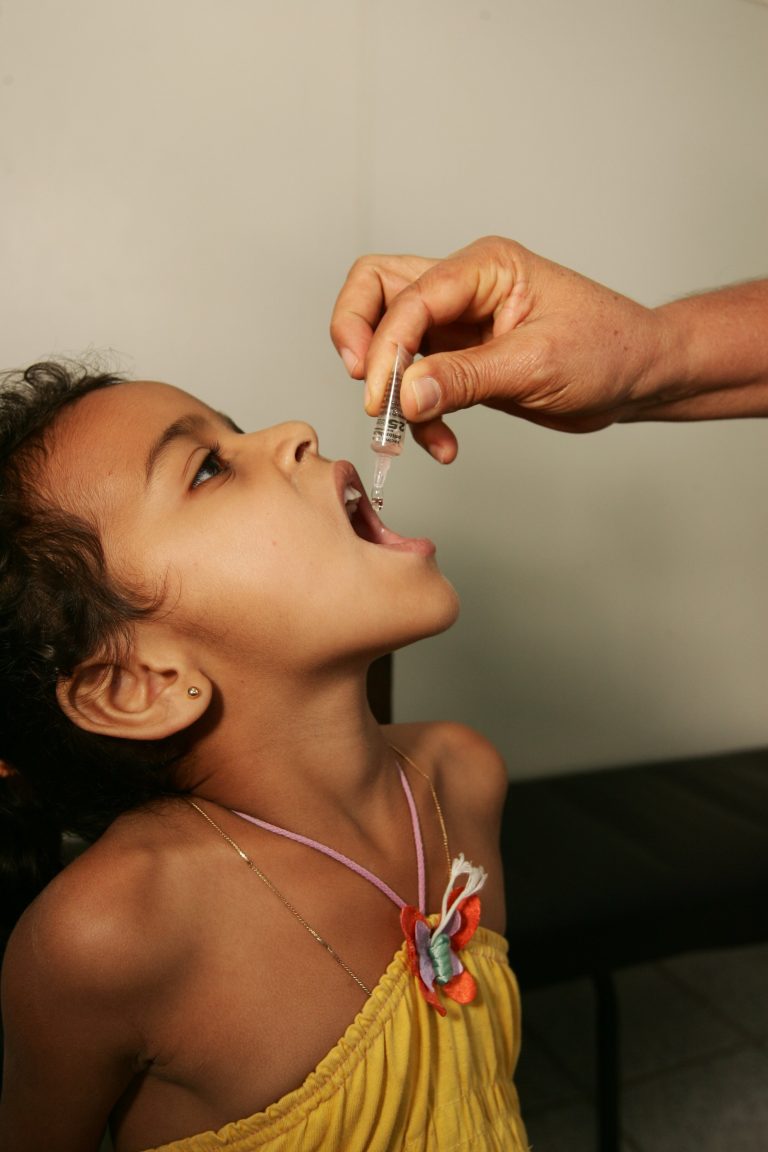
On Sept. 29, 1994, based on recommendations of the national certification committees and after review of surveillance and…

In 1994, researchers at Oxford University conducted a field trial involving a genetically engineered baculovirus, specifically one modified…
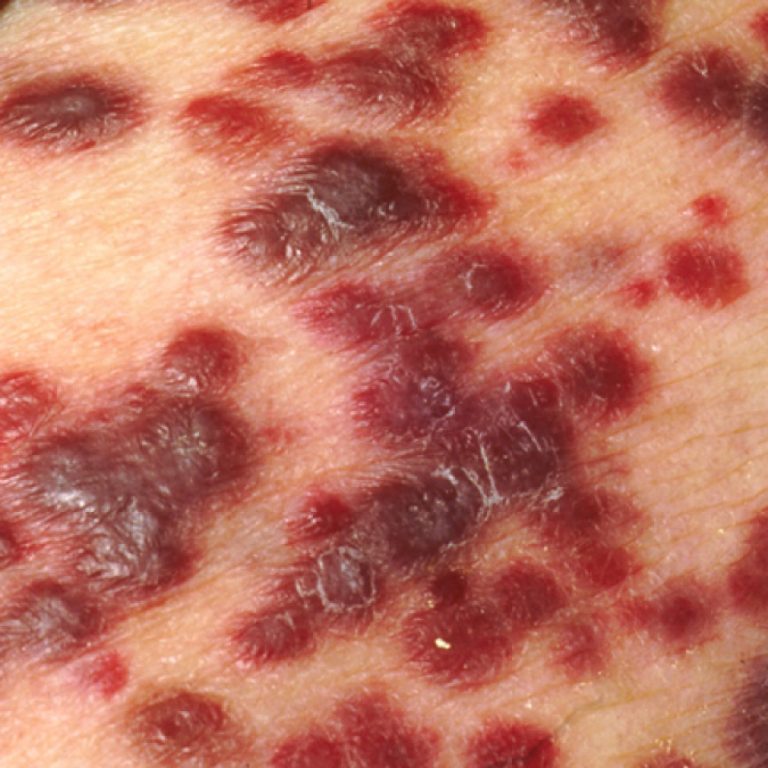
In 1994, human herpesvirus 8 (HHV-8), a causative agent for Kaposi’s sarcoma (KS), was identified. HHV-8 is also…

In 1994, Washington University – St. Louis reseacher Lee Ratern, MD and colleagues announced they had developed an…

In May 1993, the U.S. Centers for Disease Control and Prevention (CDC) responded to a Hantavirus outbreak in…
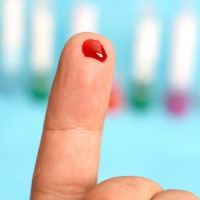
On Jun. 1, 1992, the FDA recommended that all donated blood be screened for antibodies to human immunodeficiency…

In 1990, the Global Polio Laboratory Network (GPLN) was formally established by the World Health Organization (WHO), national…

On May 22, 1989, Dr. Steven A. Rosenberg and his team at the National Cancer Institute (NCI) performed…

In 1986, while teaching a graduate course at the University of Alberta, Dr. Tyrrell found clues that might…

On Apr. 23, 1984, a National Cancer Institute (NCI) scientist, Dr. Robert C. Gallo announced his team’s discovery of…

In 1983, the U.S. Centers for Disease Control and Prevention (CDC) established the National AIDS Hotline (NAH) to…

In 1981, the U.S. Food and Drug Administration (FDA) approved a plasma-derived hepatitis B vaccine for human use….
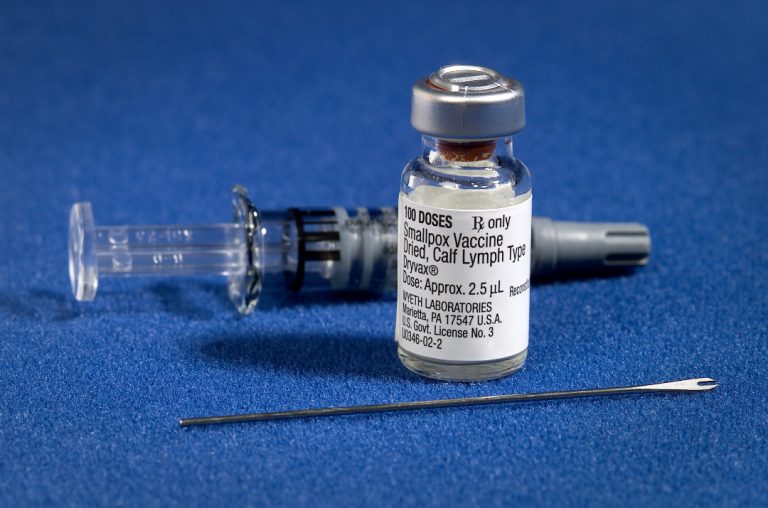
On May 8, 1980, the World Health Assembly certified the world free of naturally-occurring smallpox. Smallpox is an…

In 1980, National Cancer Institute (NCI) scientists from the Gallo group isolated human T-cell lymphotrophic virus 1 (HTLV-1)….

In 1980, McGill University researcher Dr. Kelvin Kenneth Ogilvie developed Ganciclovir to treat or prevent cytomegalovirus (CMV) infections….
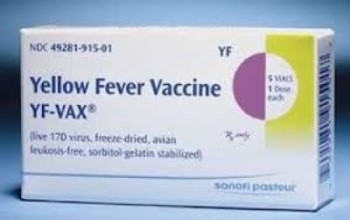
On Jan. 3, 1978, the Yellow fever vaccine (YF-Vax by Connaught) was licensed in the U.S. The Yellow…
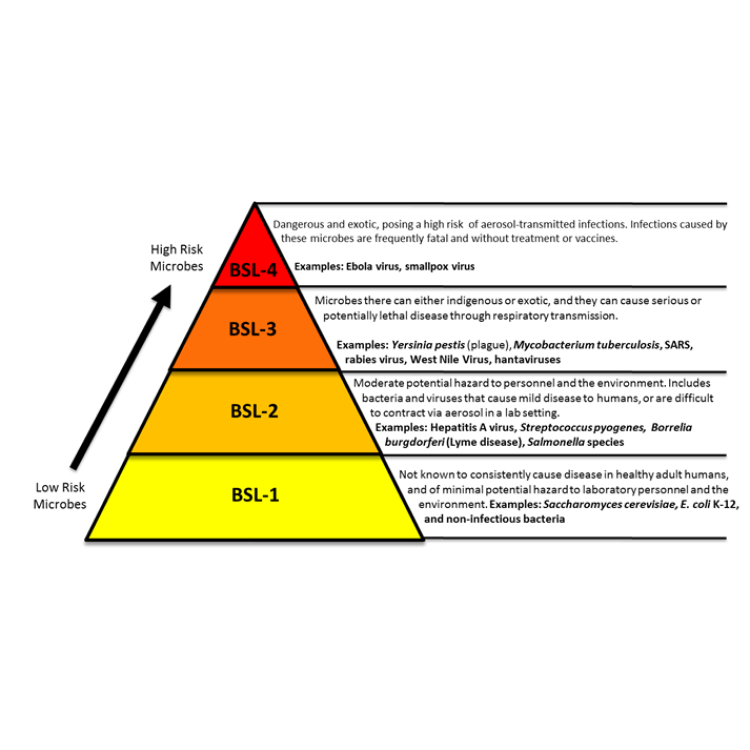
In 1978, the U.S. Centers for Disease Control and Prevention (CDC) completed construction of a new hot lab…