UC Davis researchers raceed to develop coronavirus solutions
On Mar. 20, 2020, Clinical pathologists, infectious disease physicians and scientists at the UC Davis Medical Center, California…

On Mar. 20, 2020, Clinical pathologists, infectious disease physicians and scientists at the UC Davis Medical Center, California…

On Mar. 20, 2020, Humanigen announced that the company was seeking to conduct a Phase III, randomized, controlled,…
On Mar. 20, 2020, Cardax released a white paper outlining the potential role of astaxanthin in the treatment…
On Mar. 20, 2020, Bellerophon Therapeutics announced the U.S. Food and Drug Administration (FDA) had granted emergency expanded…

On Mar. 20, 2020, Adaptive Biotechnologies and Microsoft announced they were leveraging their existing partnership mapping population-wide adaptive…

On Mar. 19, 2020, Abbott announced the FDA has issued Emergency Use Authorization (EUA) for the company’s molecular…
On Mar. 19, 2020, the Mayo Clinic announced it had significantly expanded its capacity to test clinical samples…

On Mar. 19, 2020, iBio announced its progress towards developing vaccine candidates for preventing infection from the SARS-CoV-2…

On Mar. 18, 2020, scientists from the University of Oxford’s Engineering Science Department and the Oxford Suzhou Centre…

On Mar. 18, 2020, Abbott announced that the FDA had issued Emergency Use Authorization (EUA) for the company’s…

On Mar. 18, 2020, Mateon Therapeutics reported significant progress in deploying its phase 3 clinical asset, OT-101, against…
On Mar. 18, 2020, Eli Lilly announced its scientists were partnering with the Indiana State Department of Health…

On Mar. 18, 2020, Emergent BioSolutions announced an agreement with Vaxart, whereby Emergent has agreed to utilize its…

On Mar. 17, 2020, a small study of macaques found they don’t develop a coronavirus infection the second…
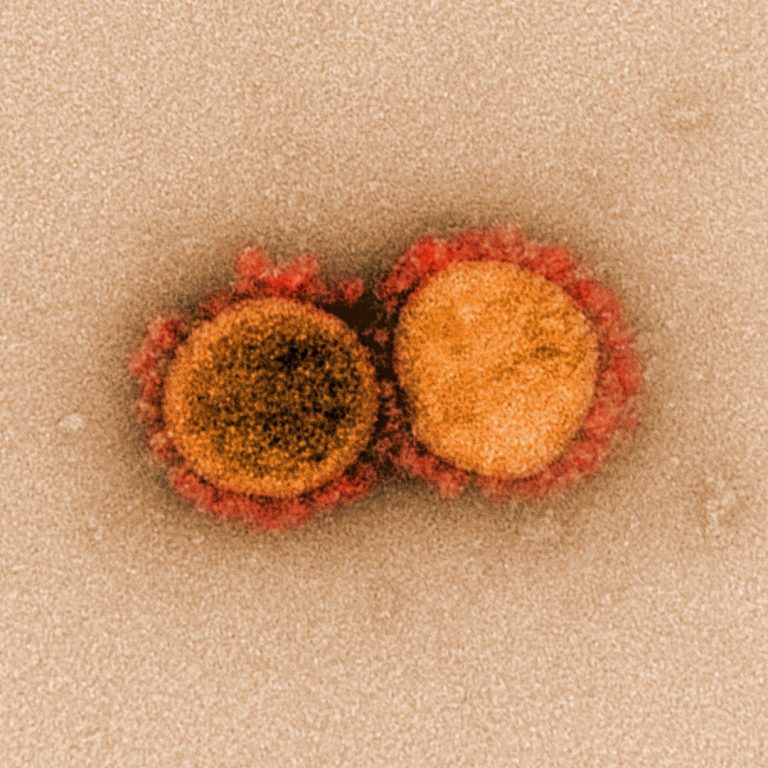
On Mar. 17, 2020, CanSino Biologics announced that its recombinant novel Coronavirus Vaccine (Adenovirus Type 5 Vector) candidate…
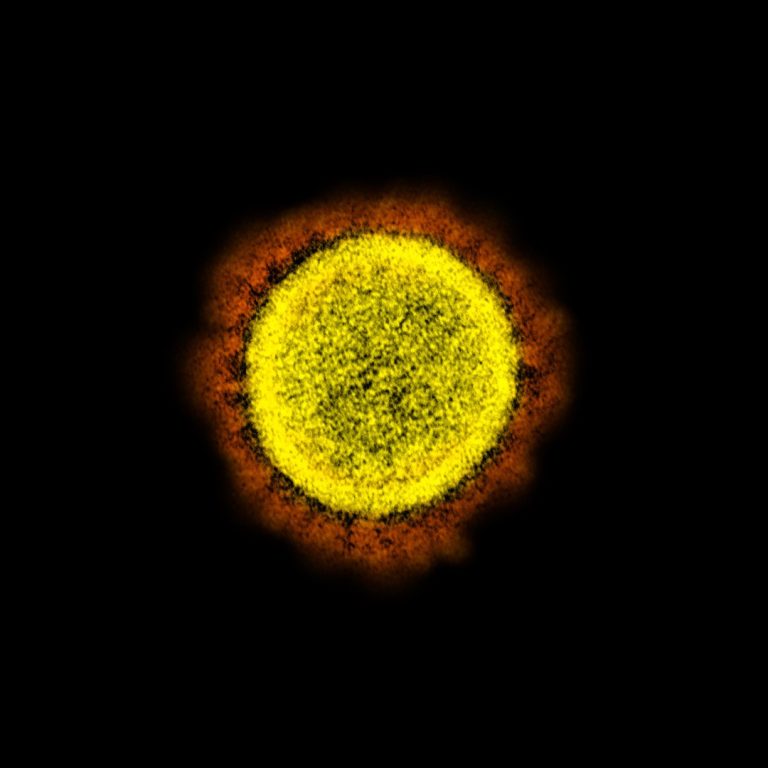
On Mar. 17, 2020, scientists reported the novel SARS-CoV-2 coronavirus that emerged in the city of Wuhan, China,…

On Mar. 17, 2020, Innovation Pharma announced details on the Material Transfer Agreement signed with a leading public…

On Mar. 17, 2020, BioMedomics announced it will be able to distribute their COVID-19 IgM-IgG Antibody Rapid Test…

On Mar. 17, 2020, BioReference Laboratories, an OPKO Health company, announced a collaboration with the New York City…

On Mar. 17, 2020, Pfizer and BioNTech SE announced the companies have agreed to a letter of intent…

On Mar. 17, 2020, Regeneron announced that its scientists had isolated hundreds of virus-neutralizing, fully human antibodies from…
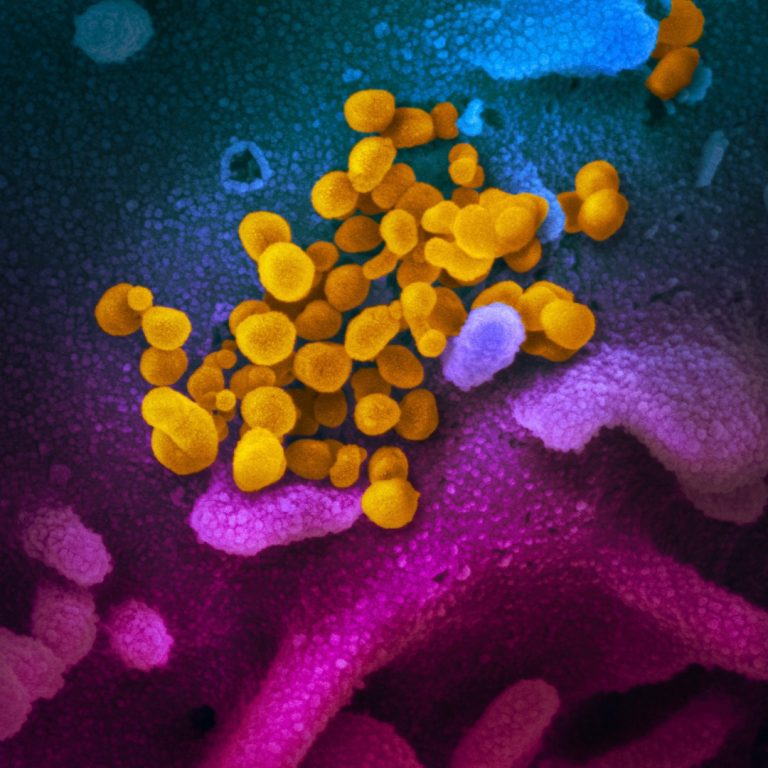
On Mar. 17, 2020, the virus that causes coronavirus disease 2019 (COVID-19) was stable for several hours to…
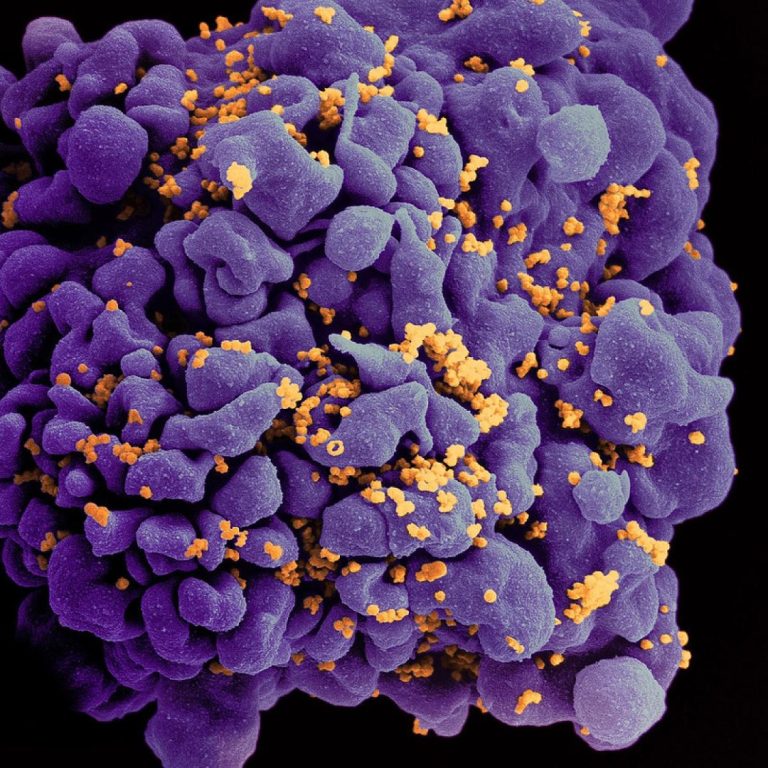
On Mar. 16, 2020, the Hormel Institute, University of Minnesota announced published research concerning remnants of ancient viruses…

On Mar. 16, 2020, the National Institutes of Health (NIH announced that a Phase 1 clinical trial evaluating…

On Mar. 16, 2020, Moderna announced that the first participant had been dosed in the Phase 1 study…

On Mar. 16, 2020, Hologic announced the U.S. Food and Drug Administration (FDA) had granted Emergency Use Authorization…

On Mar. 16, 2020, BD (Becton, Dickinson and Company) announced the companies had submitted Emergency Use Authorization requests…

On Mar. 16, 2020, PathGroup announced it had begun to offer testing for Coronavirus Disease 2019 (COVID-19). This…
On Mar. 15, 2020, the National Institutes of Health (NIH) informed its staff that it had its first…

On Mar. 15, 2020, CureVac announced that internal efforts will be focused on the development of a coronavirus…