NanoViricides announced licensing agreement with Karveer Meditech to commercialize COVID drugs
On Apr. 4, 2023, NanoViricides announced that it had executed a license agreement with Karveer Meditech, Kolhapur, India,…

On Apr. 4, 2023, NanoViricides announced that it had executed a license agreement with Karveer Meditech, Kolhapur, India,…

On Mar. 21, 2023, the Ministry of Health of the United Republic of Tanzania declared an outbreak of…
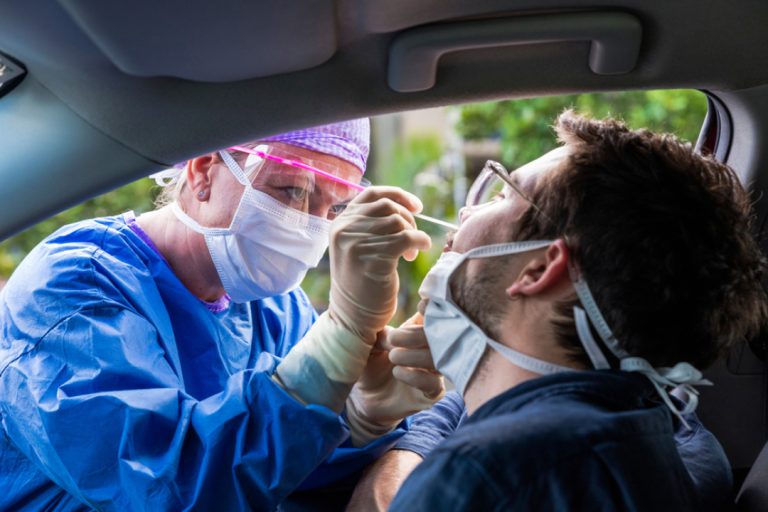
On Mar. 21, 2023, Quest Diagnostics announced that it had introduced two innovative Post-COVID-19 panels. These laboratory tests…

On Feb. 26, 2023, the Cambodia International Health Regulations (IHR) National Focal Point (NFP) reported one confirmed case…
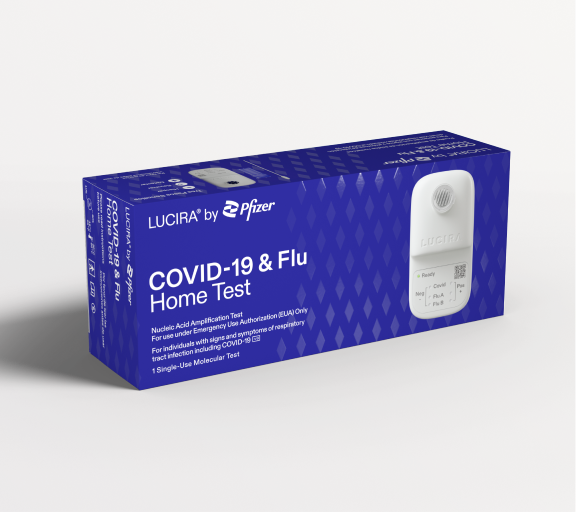
On Feb. 24, 2023, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for…
On Feb. 15, 2023, it was reported that a 53-year-old man in Germany had become at least the…
On Feb. 14, 2023, scientists from the National Institute of Allergy and Infectious Diseases announced they had developed…

On Feb. 13, 2023, Equatorial Guinea today confirmed its first-ever outbreak of Marburg virus disease. Preliminary tests carried…

On Feb. 8, 2023, as bird flu continues to circle the globe, a new report suggested that the…
On Feb. 8, 2023, BD announced that it had received Emergency Use Authorization from the U.S. Food and…

On Feb. 7, 2023, the U.S. Department of Agricultureメs Research Service announced that it was implementing a $300…
On Feb. 2, 2023, a National Institutes of Health research group with extensive experience studying ebolavirus countermeasures successfully…
On Jan. 11, 2023, Uganda declared the end of the Ebola disease outbreak caused by Sudan ebolavirus, less…

On Jan. 11, 2023, the U.S. Food and Drug Administration reported that a single booster dose with an…

On Jan. 10, 2023, the WHO announced that an estimated 5 million children died beforecoronavirus_omicron_illustrationeir fifth birthday and…
On Jan. 9, 2023, BD (Becton, Dickinson) and CerTest Biotec have announced Emergency Use Authorization (EUA) from the…

On Jan. 4, 2023, a National Institutes of Health funded study reported that moderate levels of two outdoor…

On Dec. 20, 2022, the University of Oxford announced study results using data from 7 million people notified…
On Dec. 14, 2022, the National Institutes of Health announced that two randomized, placebo-controlled trials evaluating three Ebola…

On Dec. 8, 2022, Moderna announced it had received emergency use authorization from the U.S. Food and Drug…

On Dec. 7, 2022, Novavax announced that Health Canada had approved a supplement to a New Drug Submission…
On Dec. 7, 2022, Pfizer announced that the U.S. Food and Drug Administration (FDA) had accepted for priority…

On Nov. 22, 2022, the U.S. Food and Drug Administration approved the investigational gene therapy etranacogene dezaparvovec or…

On Nov. 21, 2022, the National Institutes of Health awarded more than $12 million to three institutions for…

On Nov. 18, 2022, Novavax announced that Health Canada had granted expanded authorization for Nuvaxovid (COVID-19 Vaccine (Recombinant…
On Nov. 15, 2022, Roche announced that the U.S. Food and Drug Administration had granted Emergency Use Authorization…

On Nov. 9, 2022, researchers at the University of Missouri announced they had identified the highly prevalent, specific…

On Nov. 9, 2022, Novavax announced that the Medicines and Healthcare products Regulatory Agency (MHRA) in the United…

On Nov. 3, 2022, Health Canada authorized an adapted version of the Moderna Spikevax COVID-19 vaccine that targets…

On Nov. 2, 2022, AIM ImmunoTech announced that the U.S. Food and Drug Administration had granted Orphan Drug…