Mink at affected Oregon farm negative for SARS-CoV-2, wildlife surveillance continued
On Dec. 23, 2020, recent tests confirmed mink that tested positive for SARS-CoV-2 at an Oregon farm in…

On Dec. 23, 2020, recent tests confirmed mink that tested positive for SARS-CoV-2 at an Oregon farm in…
On Dec. 22, 2020, Cocrystal Pharma announced the selection of CDI-45205 as the lead compound for further development…

On Dec. 22, 2020, the NIH researchers announced they had isolated a set of promising, tiny antibodies, or…

On Dec. 22, 2020, Sorrento Therapeutics announced that an Emergency Use Authorization (EUA) Application had been submitted to…

On Dec. 21, 2020, the NIH announced that it had awarded eight research grants to develop approaches for…
On Dec. 21, 2020, the U.S. National Institutes of Health (NIH) announced a study published in the Proceedings…

On Dec. 21, 2020, Pfizer and BioNTech announced that the Committee for Medicinal Products for Human Use (CHMP)…

On Dec. 21, 2020, Pfizer and BioNTech announced that the European Commission (EC) had granted a conditional marketing…

On Dec. 18, 2020, the FDA issued an emergency use authorization (EUA) for the second vaccine for the…
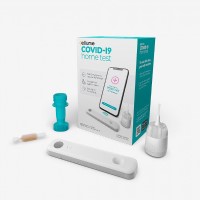
On Dec. 15, 2020, the FDA granted emergency use authorization (EUA) for the first over-the-counter (OTC) fully at-home…

On Dec. 15, 2020, TGen, an affiliate of City of Hope announced that it had identified a specific…

On Dec.14, 2020, neuroscientists at University College London (UCL) announced they had found no significant association between COVID-19…

On Dec. 14, 2020, Codagenix and the Serum Institute of India announced that a Phase 1 clinical trial…
On Dec. 11, 2020, the FDA issued the first emergency use authorization (EUA) for a vaccine for the…

On Dev. 11, 2020, the University of Oxford reported that data from the National COVID-19 Infection Survey, done…

On Dec. 11, 2020, the USDA’s National Veterinary Services Laboratories announced the first confirmed case of SARS-CoV-2 (the…

On Dec. 10, 2020, Dynavax Technologies announced that the European Medicines Agency (EMA) Committee for Medicinal Products for…

On Dev. 8, 2020, University of Oxford and AstraZeneca researchers present a pooled analysis of Phase 3 trials…
On Dec. 8, 2020, University of Alberta virologist Michael Houghton was awarded a share of the 2020 Nobel…

On Dec. 2, 2020, Roche announced that its Elecsysᆴ Anti-SARS-CoV-2 S antibody test had received Emergency Use Authorization…

On Dec. 1, 2020, VBI Vaccines announced the submission of a Biologics License Application (BLA) to the FDA…

On Nov. 25, 2020, the National Institute of Allergy and Infectious Diseases (NIAID), one of the National Institutes…

On Nov. 30, 2020, Innovation Pharmaceuticals announced additional independent preliminary laboratory research suggests Brilacidin, the Company’s flagship defensin…

On Nov. 25, 2020, the National Institute of Allergy and Infectious Diseases (NIAID), one of the National Institutes…
On Nov. 24, 2020, preclinical study by the National Institutes of Health (NIH) suggested FDA-approved tetracycline-based antibiotics may…

On Nov. 24, 2020, published data from researchers at Mayo Clinic found that physical separation reduced the exposure…

On Nov. 20, 2020, the ‘European Food Safety Authority reported that within the past month more than 300…

On Nov. 30, 2020, Hologic announced that the FDA had approved a diagnostic claim for its HIV-1 (human…

On Nov. 20, 2020, researchers at the Broad Institute of MIT and Harvard announced they had developed a…

On Nov. 19, 2020, the University of Oxford announced that the ChAdOx1 nCov-2019 coronavirus vaccine had been shown…