WHO announced influenza A(H1N2) variant virus infection in the Great Britain and Northern Ireland
On Dec. 1, 2023, the World Health Organization (WHO) announced that the International Health Regulations National Focal Point…
On Dec. 1, 2023, the World Health Organization (WHO) announced that the International Health Regulations National Focal Point…

On Nov. 16, 2023, Vertex and CRISPR Therapeutics announced that the United Kingdom (U.K.) Medicines and Healthcare products…
On Oct. 18, 2023, Novavax announced that the Medicines and Healthcare products Regulatory Agency (MHRA) in the United…

On Aug. 7, 2023, the United Kingdom Health and Security Agency announced the Vaccine Development and Evaluation Centre…

On Jul. 14, 2023, the UK Animal and Plant Health Agency reported that additional asymptomatic human detections of…

On May 30, 2023, the United Kingdom of Great Britain and Northern Ireland reported to the World Health…
On Jan. 5, 2023, BioNTech announced that the Company had signed a Memorandum of Understanding with the Government…

On Jan. 5, 2023, Novavax announced that Spain’s Public Health Commission, Italy’s Ministry of Health, and France’s Haute…

On Dec. 21, 2022, Moderna announced the the finalization of a strategic partnership with the United Kingdom (UK)…

On Nov. 9, 2022, Novavax announced that the Medicines and Healthcare products Regulatory Agency (MHRA) in the United…
On Sept. 27, 2022, the National Institutes of Health (NIH) announced that a large international study had confirmed…

On Sept. 27, 2022, Novavax announced that an initial one million doses of Nuvaxovid (NVX-CoV2373) COVID-19 vaccine were…

On Aug. 26, 2022, Novavax announced that the Medicines and Healthcare products Regulatory Agency (MHRA) in the United…
On Jun. 2, 2022, Novavax announced the submission of a request to the Medicines and Healthcare products Regulatory…

On May 25, 2022, Novavax announced it was participating in a stage of the COVID-19 Vaccine Schedule Combinations…
On May 21, 2022, the World Health Organization (WHO) reported that since May 13, 2022, cases of mpox…
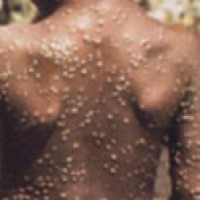
On May 7, 2022, WHO was informed of a confirmed case of monkeypox in an individual who travelled…

On Feb. 24, 2022, Novavax shared extended analysis from its pivotal Phase 3 clinical trial conducted in the…
On Feb. 23, 2022, Sanofi and GSK announced that they planned to submit data from both their booster…

On Feb. 3, 2022, Novavax announced that the Medicines and Healthcare products Regulatory Agency had granted conditional marketing…

On Jan. 31, 2022, Novavax announced that it had submitted a request to the U.S Food and Drug…

On Jan. 18, 2022, Merck and Ridgeback Biotherapeutics announced the signing of a long-term supply agreement with the…

On Jan. 6, 2022, two new studies published in the journal Current Biology showed that environmental DNA (eDNA)…

On Dec. 22, 2021, Merck and Ridgeback Biotherapeutics announced that the United Kingdom Government agreed to purchase an…

On Dec. 1, 2021, Moderna announced a revised supply agreement with the UK government for up to 60…

On Oct. 27, 2021, Novavax announced the completion of its rolling regulatory submission to the U.K. Medicines and…

On Oct. 26, 2021, Axcella Therapeutics announced a new clinical program to investigate AXA1125 as a potential treatment…

On Aug. 20, 2021, Regeneron announced that the United Kingdom’s (UK) Medicines and Healthcare products Regulatory Agency had…

On Aug. 19, 2021, the University of Oxford reported that obtaining two vaccine doses remained the most effective…

On Aug. 4, 2021, Novavax announced that it had reached an agreement with the European Commission (EC) for…