Novavax and Serum Institute of India announced WHO Emergency Use listing for NVX-CoV2373 COVID-19 vaccine
On Dec. 17, 2021, Novavax and SK bioscience announced that the World Health Organization (WHO) had granted Emergency…

On Dec. 17, 2021, Novavax and SK bioscience announced that the World Health Organization (WHO) had granted Emergency…

On Dec. 15, 2021, Twist Bioscience announced that synthetic RNA positive controls were available for the SARS-CoV-2 Omicron…

On Dec. 14, 2021, Pfizer announced final results from an analysis of all 2,246 adults enrolled in its…

On Dec. 13, 2021, the Coalition for Epidemic Preparedness Innovations, and Affinivax announced a partnership to advance the…

On Dec. 8, 2021, the U.S. Food and Drug Administration issued an emergency use authorization for AstraZenecaメs Evusheld…

On Dec. 8, 2021, Pfizer and BioNTech announced results from an initial laboratory study demonstrating that serum antibodies…

On Dec. 8, 2021, PerkinElmer announced the research use only launch of the NEXTFLEXᆴ Variant-Seqル SARS-CoV-2 Kit v2…

On Dec. 7, 2021, Rockefeller University scientists announced a study had demonstrated the therapeutic potential of an unusual…

On Dec. 7, 2021, Arbutus Biopharma, X-Chem, and Proteros announced that Arbutus had identified several molecules that inhibit…

On Dec. 5, 2021, Sorrento Therapeutics announced the peer-reviewed publication of a series of novel SARS-CoV-2 MPro inhibitors…

On Dec. 5, 2021, Roche announced that its planned to launch the SARS-CoV-2 & Flu A/B Rapid Antigen…

On Dec. 3, 2021, Roche and TIB Molbiol announced they had added three additional Research Use Only test…
On Dec. 1, 2021, T2 Biosystems announced that its T2SARS-CoV-2 Panel detected the Omicron COVID-19 variant (B.1.1.529). The…

On Dec. 1, 2021, Fulgent Genetics confirmed that the Companyメs RT-PCR test for SARS-CoV-2, the virus that causes…
On Nov. 30, 2021, Inovio Pharma announced the company was rapidly moving to evaluate its COVID-19 DNA vaccine…

On Nov. 29, 2021, Thermo Fisher Scientific confirmed that its polymerase chain reaction (PCR) TaqPath COVID-19 Combo Kit…
On Nov. 29, 2021, Hologic announced that its three SARS-CoV-2 tests all detect the recently emerged Omicron variant of…

On Nov. 26, 2021, Merck provided an update on the MOVe-OUT study of molnupiravir, an investigational oral antiviral…

On Nov. 26, 2021, the World Health Organization announced that the Technical Advisory Group on SARS-CoV-2 Virus Evolution…

On Nov. 23, 2021, the World Health Organization’s COVID-19 Technology Access Pool and the Medicines Patent Pool finalized…
On Nov. 22, 2021, Tonix Pharmaceuticals announced the publication of ‘Sangivamycin is highly effective against SARS-CoV-2 in vitro…

On Nov. 19, 2021, the U.S. Food and Drug Administration (FDA) announced that it had extended the emergency…
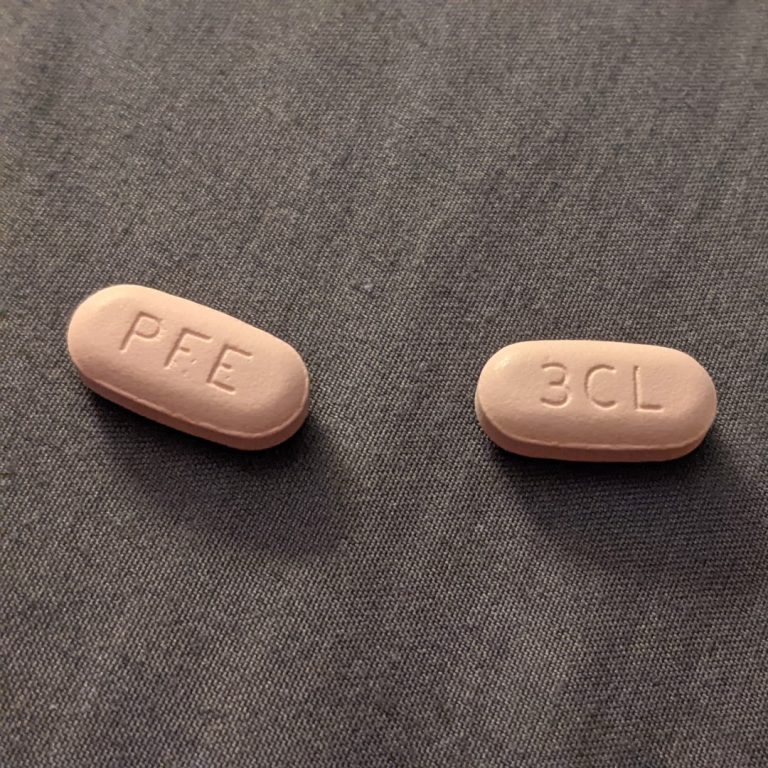
On Nov. 18, 2021, Pfizer announced an agreement with the U.S. government to supply 10 million treatment courses…

On Nov. 16, 2021, Pfizer announced it had submitted an Emergency Use Authorization (EUA) of its investigational oral…

On Nov. 10, 2021, Meridian Bioscience announced that their Revogeneᆴ SARS-CoV-2 assay was granted Emergency Use Authorization by…

On Nov. 9, 2021, Moderna announced that it has submitted for a variation to the conditional marketing authorization…

On Nov. 8, 2021, Cocrystal Pharma announced that its SARS-CoV-2 main protease inhibitors showed potent in vitro pan-viral…

On Nov. 5, 2021, the USDA’s (USDA) National Veterinary Services Laboratories announced confirmation of SARS-CoV-2 in two spotted…

On Nov. 5, 2021, scientists at the National Institutes of Health announced they had found that a process…

On Nov. 5, 2021, Pfizer announced it was investigational novel COVID-19 oral antiviral candidate, PAXLOVID, significantly reduced hospitalization…