Pfizer and BioNTech received Health Canada authorization for XBB.1.5-adapted monovalent COVID-19 vaccine
On Sept. 28, 2023, Pfizer and BioNTech that Health Canada had authorized the companies’ Omicron XBB.1.5-adapted monovalent COVID-19…

On Sept. 28, 2023, Pfizer and BioNTech that Health Canada had authorized the companies’ Omicron XBB.1.5-adapted monovalent COVID-19…

On Sept. 27, 2023, Gritstone bio announced that it was awarded a contract by the Biomedical Advanced Research…

On Sept. 22, 2023, a new study showed the isolation and sequencing of more than a century-old RNA…

On Sept. 11, 2023, Pfizer and BioNTech announced that the U.S. Food and Drug Administration (FDA) approved the…

On Aug. 30, 2023, Pfizer and BioNTech announced that the Committee for Medicinal Products for Human Use (CHMP)…
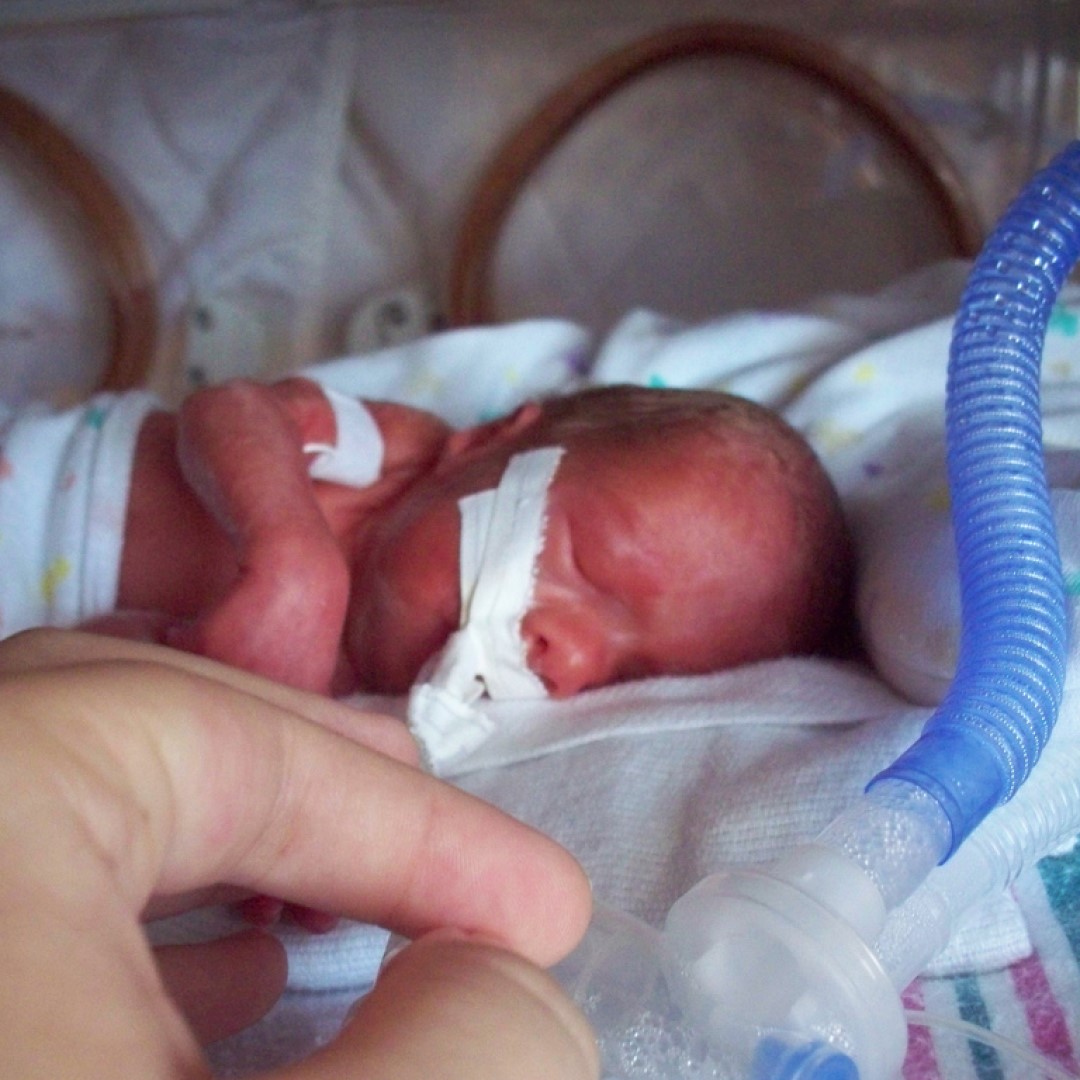
On Aug. 11, 2023, a study funded by the National Institute of Allergy and Infectious Diseases (NIAID) reported…

On Jul. 25, 2023, U.S. National Science Foundation-supported research, led by Joshua Rosenthal of the Marine Biological Laboratory…

On Jul. 18, 2023, scientists reported that they had extracted, sequenced, and analyzed historical RNA from muscle and…
On Apr. 27, 2023, a NIAID-led Phase 2 trial compared two Pfizer bivalent mRNA COVID-19 boosters found that…
On Apr. 25, 2023, the U.S. FDA approved Qalsody (tofersen) to treat patients with amyotrophic lateral sclerosis (ALS)…

On Mar. 30, 2023, Moderna and the Government of the Republic of Kenya announced they had finalized an…
On Mar. 15, 2023, scientists at Texas A&M announced that a research study: ‘RNA interference is essential to…

On Feb. 17, 2023, Moderna announced that Health Canada had authorized the use of its Omicron-targeting bivalent COVID-19…

On Feb. 9, 2023, the National Institutes of Health (NIH) announced that a new large-scale genetic analysis found…
On Jan. 17, 2023, Moderna announced positive topline data from its ConquerRSV Phase 3 pivotal efficacy trial of…
On Jan. 5, 2023, BioNTech announced that the Company had signed a Memorandum of Understanding with the Government…

On Dec. 23, 2022, BioNTech and Fosun Pharmaceutical announced that they had received the certificates of registration as…

On Dec. 21, 2022, Moderna announced the the finalization of a strategic partnership with the United Kingdom (UK)…

On Dec. 16, 2022, Moderna announced that the European Medicines Agency’s Committee for Medicinal Products for Human Use…

On Dec. 9, 2022, Pfizer and BioNTech announced the companies had received Fast Track Designation from the U.S….

On Dec. 8, 2022, Moderna announced it had received emergency use authorization from the U.S. Food and Drug…

On Nov. 16, 2022, Pfizer and BioNTech announced that the companies had initiated a Phase 1 study to…

On Nov. 15, 2022, Moderna reported findings from a Phase II/III clinical trial where bivalent Omicron-targeting booster candidates,…

On Nov. 10, 2022, Pfizer and BioNTech announced a booster dose of their Omicron BA.4/BA.5-adapted bivalent COVID-19 vaccine…
On Nov. 9, 2022, Novavax announced that the Medicines and Healthcare products Regulatory Agency (MHRA) in the United…

On Nov. 3, 2022, Health Canada authorized an adapted version of the Moderna Spikevax COVID-19 vaccine that targets…

On Oct. 31, 2022, Moderna announced that it had received approval from the Ministry of Health, Labour and…

On Oct. 19, 2022, the U.S. Food and Drug Administration (FDA) recommended the use of the Novavax COVID-19…

On Oct. 19, 2022, Moderna announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human…

On Oct. 19, 2022, Moderna announced new clinical data on its bivalent Omicron-containing booster, mRNA-1273.214. Ninety days after…