NIH delivered $43 million to support convalescent plasma clinical trial
On Aug. 18, 2020, the National Institutes of Health awarded Albert Einstein College of Medicine and Montefiore a…

On Aug. 18, 2020, the National Institutes of Health awarded Albert Einstein College of Medicine and Montefiore a…

On Aug. 17, 2020, Scientisfic American reported that Costa Rican scientists at the Clodomiro Picado Institute have inoculated…

On Aug. 11, 2020, LabCorpᆴ announced details of a no charge antibody testing program in response to federal…

On Aug. 5, 2020, AXIM Biotechnologies announced the development, patent filing and Emergency Use Approval (EUA) filing of…
On Jul. 15, 2020, BioAegis Therapeutics announced that it received regulatory clearance from The Spanish Agency for Medicines…

On Jul. 8, 2020, Kleo Pharmaceuticals announced that it had received a $5 million grant from the Bill…

On Jul. 7, 2020, Cerus announced study results that demonstrated the INTERCEPT Blood System inactivates SARS-CoV-2, the causative…

On Jul. 6, 2020, ADMA Biologics announced the commencement of operations and initiation of collections at its newest…

On Jun. 25, 2020, LabCorpᆴ announced the launch of a new test that can be used to assess…

On Jun. 1, 2020, U.S. Dept. of Defense announced it had begun an effort to collect 8,000 donated…

On May 21, 2020, ADMA Biologics announced it had commenced the collection of convalescent plasma through its wholly-owned…

On May 21, 2020, BioAegis Therapeutics announced that the National Institute of Health Clinical Center began measuring patient…
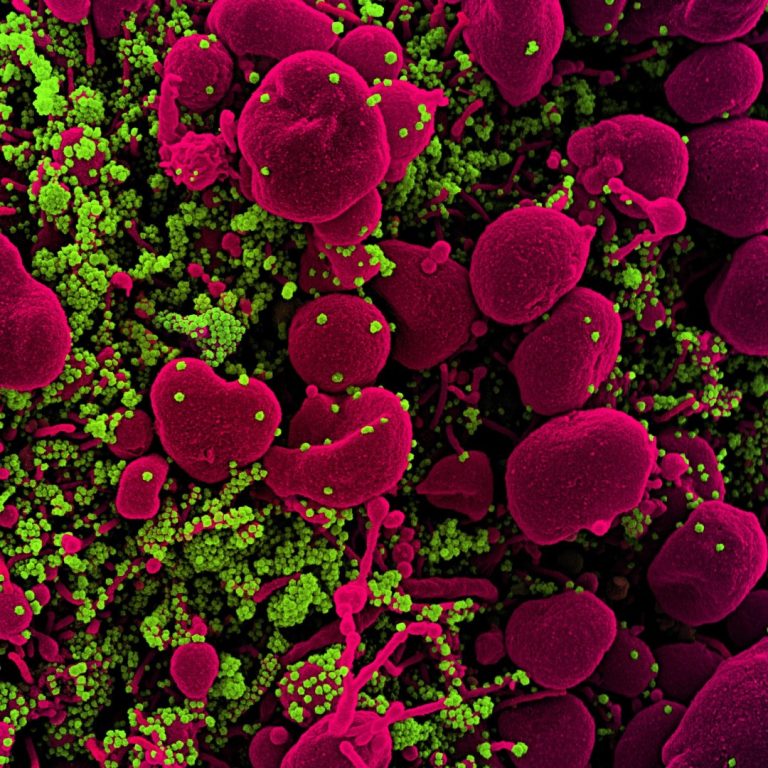
On May 11, 2020, Arbutus Biopharma announced the establishment of a new research initiative focused on the identification…

On May 8, 2020, the CoVIg-19 Plasma Alliance was announced, an unprecedented partnership of the world’s leading plasma…

On May 7, 2020, the CoVId-19 Plasma Alliance, an unprecedented plasma industry collaboration recently established to accelerate the…
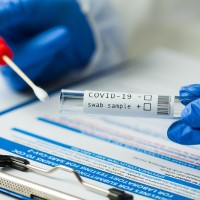
On May 5, 2020, ACON Laboratories announced the availability of its SARS-COV-2 IgG/IgM Rapid Test. ACONメs test is…

On May 4, 2020, Cerus announced FDA regulatory approval for manufacture of INTERCEPT plasma with a new, alternative…

On May 4, 2020, Mayo Clinic was awarded a $26 million contract from the Biomedical Advanced Research and…

On Apr. 30, 2020, The California Institute for Regenerative Medicine (CIRM) awarded $750,000 to City of Hopeメs John…

On Apr. 29, 2020, researchers at Montefiore Health System and Albert Einstein College of Medicine and NYU Langone…

On Apr. 27, 2020, BioAegis Therapeutics announced it had published gene expression data in animal studies where recombinant…

On Apr. 24, 2020, the governing Board of the California Institute for Regenerative Medicine (CIRM) awarded $749,999 to…

On Apr. 17, 2020, Roche announced the development and upcoming launch of its Elecsys Anti-SARS-CoV-2 serology test to…

On Apr. 14, 2020, NantKwest and ImmunityBio announced discussions with the FDA for vaccines and therapeutics to combat…

On Apr. 13, 2020, a new serology test from Mayo Clinic Laboratories that is used to identify the…

On Apr. 9, 2020, Chembio Diagnostics has been selected for use in a Stony Brook Medicine effort to…

On Apr. 8, 2020, CSL Behring and SAB Biotherapeutics announced a partnership to combat the coronavirus pandemic with…

On Apr. 6, 2020, Biotest, BPL Group, LFB, and Octapharma joined an alliance formed by CSL Behring and…

On Apr. 3, 2020, the FDA announced that the Mayo Clinic Mayo Clinic will be the lead institution…

On Apr. 3, 2020, XBiotech and BioBridge Global announced a collaboration to participate in a FDA investigational program…