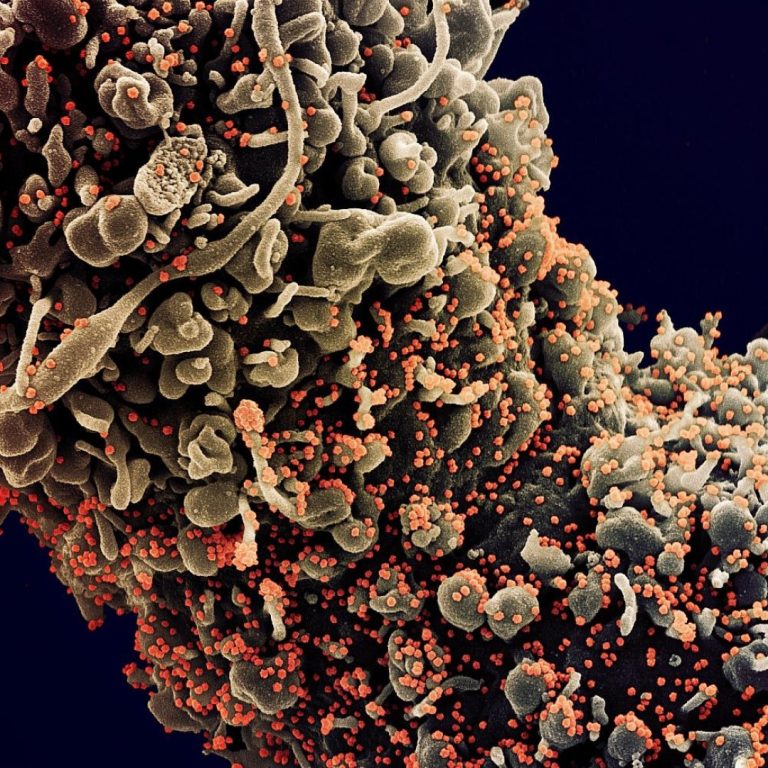
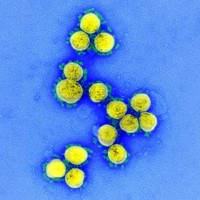
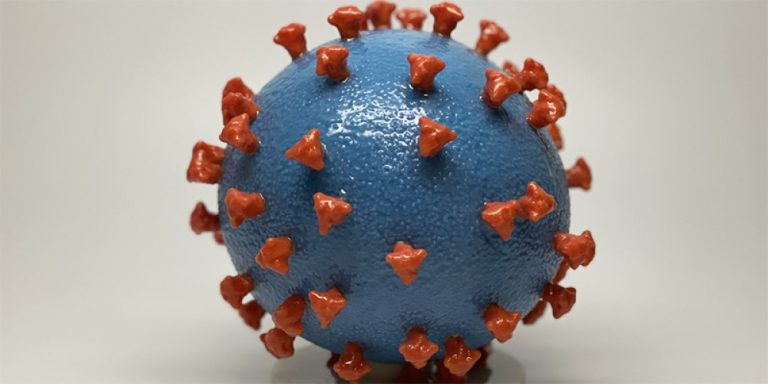
Cumberland Pharmaceuticals announced initiative to expand availability of Caldolor to treat fevers associated with Coronavirus Infections
On Apr. 1, 2020, Cumberland Pharma announced a national initiative to support hospitals and clinics that use Caldolor…