CureVacメs COVID-19 vaccine candidate demonstrated protection against SARS-CoV-2 B1351 variant
On Mar. 23, 2021, CureVac announced publication of preclinical data demonstrating that their COVID-19 vaccine candidate, CVnCoV, protected…

On Mar. 23, 2021, CureVac announced publication of preclinical data demonstrating that their COVID-19 vaccine candidate, CVnCoV, protected…

On Mar. 23, 2021, Roche confirmed positive topline results from the largest trial to date assessing a COVID-19…

On Mar. 22, 2021, CureVac announced plans to expand and further specify the protocols of its ongoing late-stage…

On Mar. 22, 2021, the NIH announced that results from a large clinical trial in the U.S. and…

On Mar. 22, 2021, Oxford University announced that a Phase III study of the Oxford-AstraZeneca coronavirus vaccine conducted…

On Mar. 22, 2021, AIM ImmunoTech announced that the Institutional Review Board (IRB) of Roswell Park Comprehensive Cancer…

On Mar. 22, 2021, Moderna announced that the Philippines had secured 7 million additional doses of COVID-19 Vaccine…

On Mar. 21, 2021, Cepheid announced it had received Emergency Use Authorization from the U.S. Food & Drug…

On Mar. 19, 2021, the FDA issued an emergency use authorization to Tiger Tech Solutions for the first…

On Mar. 18, 2021, XPhyto and 3a-diagnostics announced the European approval of its point-of-care SARS-CoV-2 (COVID-19) RT-PCR test…
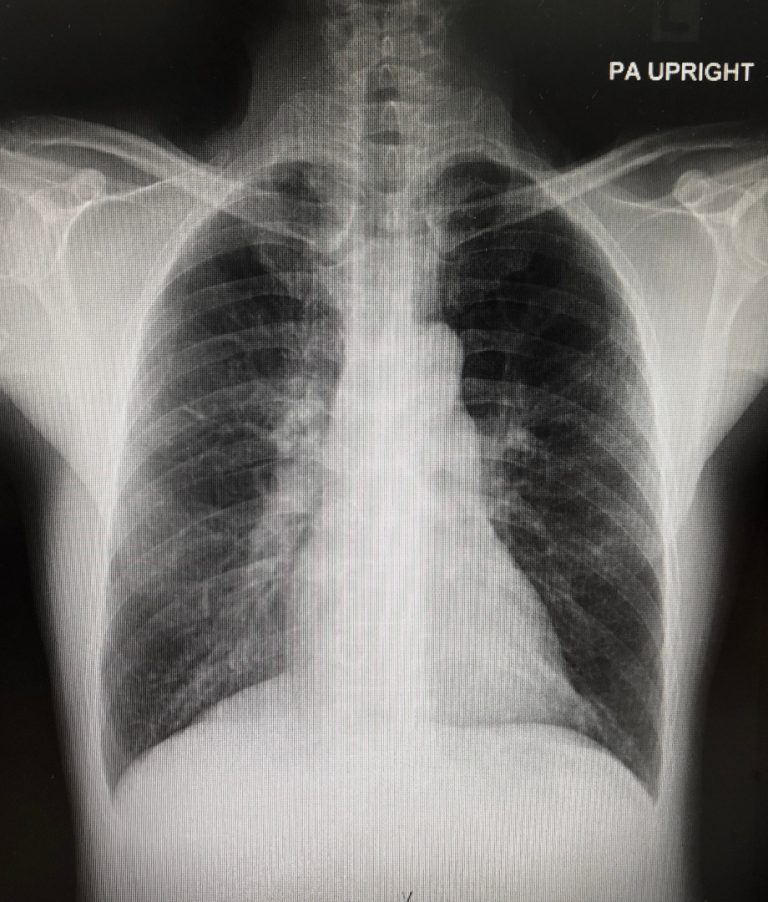
On Mar. 18, 2021, Incyte announced results from the Phase 3 DEVENT study evaluating the efficacy and safety…

On Mar. 18, 2021, AstraZeneca announced that the Medicines and Healthcare products Regulatory Agency (MHRA) and European Medicines…
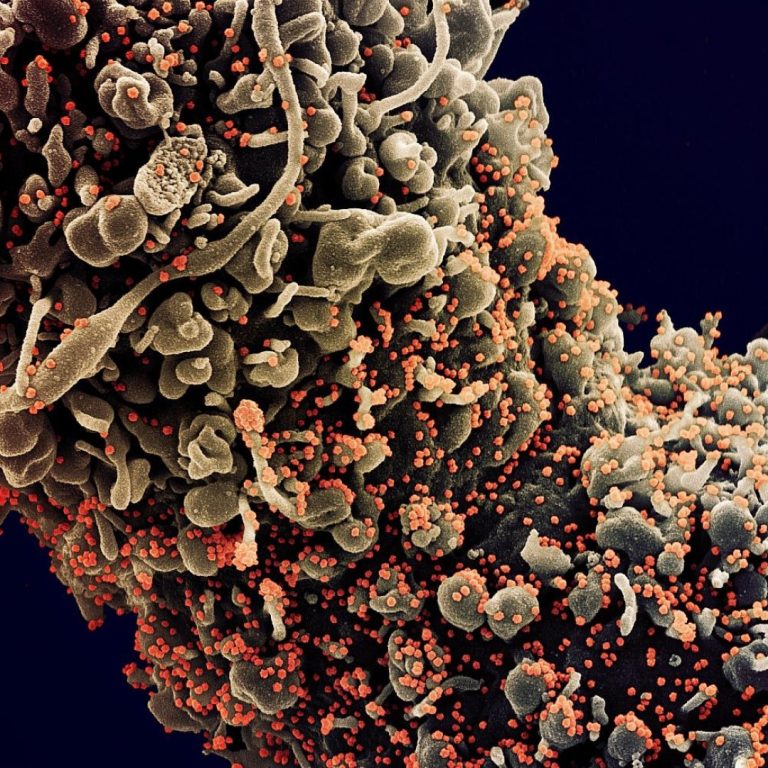
On Mar. 17, 2021, the WHO reported that the AstraZeneca COVID-19 vaccine (including Covishield) continued to have a…

On Mar. 17, 2021, the FDA granted marketing authorization of the BioFire Respiratory Panel 2.1 (RP2.1), a diagnostic…

On Mar. 17, 2021, Tonix Pharma announced preliminary results following vaccination of non-human primates with TNX-1800 (modified horsepox…

On Mar. 16, 2021, Roche announced the launch of the cobasᆴ SARS-CoV-2 Variant Set 1 Test to detect…

On Mar. 16, 2021, AstraZeneca announced it had modified an existing agreement with the US Government to supply…
On Mar. 16, 2021, research from the WHO and partners showed that the COVID-19 pandemic was severely affecting…

On Mar. 16, 2021, Moderna announced that the first participants had been dosed in the Phase 2/3 study,…

On Mar. 15, 2021, Resverlogix announced the publishing of a new article titled: モBromodomain and extraterminal protein inhibitor,…

On Mar. 15, 2021, ImmunityBio announced it had met the safety requirements for the first 12 participants in…

On Mar. 15, 2021, Moderna announced that the first participants had been dosed in the Phase 1 study…

On Mar. 15, 2021, Altimmune announced additional preclinical data for its single dose intranasal COVID-19 vaccine candidate, AdCOVID….

On Mar. 12, 2021, the WHO listed the COVID-19 vaccine Ad26.COV2.S, developed by Janssen (Johnson & Johnson), for…

On Mar. 12, 2021, Johnson & Johnson announced that the World Health Organization (WHO) had issued Emergency Use…

On Mar. 11, 2021, Rigel Pharma announced the completion of patient enrollment in a multi-center Phase 2 clinical…

On Mar. 11, 2021, Altimmune announced that it had expanded its previously-announced AdCOVID manufacturing collaboration with Lonza. Under…

On Mar. 11, 2021, Novavax announced final efficacy of 96.4% against mild, moderate and severe disease caused by…

On Mar. 11, 2021, Johnson & Johnson announced that the Committee for Medicinal Products for Human Use (CHMP)…

On Mar. 11, 2021, Johnson & Johnson announced that the European Commission (EC) had granted a Conditional Marketing…