University at Buffalo to receive $50 million for biofabrication and imaging research hub
On Nov. 25, 2025, Governor Kathy Hochul announced the University at Buffalo (UB) will receive $50 million from…

On Nov. 25, 2025, Governor Kathy Hochul announced the University at Buffalo (UB) will receive $50 million from…

On Nov. 17, 2025, Sandoz announced that TYRUKO® (natalizumab-sztn) is available to patients in the US. Developed by…
On Oct. 13, 2025, researchers from Texas A&M University announced they have identified two estrogens, estradiol and estriol,…

On Sept. 13, 2024, Genentech announced that the U.S. Food and Drug Administration (FDA) had approved Ocrevus Zunovo™…
On Aug. 24, 2023, the U.S. Food and Drug Administration approved Sandoz’s Tyruko (natalizumab-sztn), the first biosimilar to…

On Jan. 24, 2022, Atara Biotherapeutics, a leader in T-cell immunotherapy, announced that it had leveraged its novel…

On Dec. 2, 2021, researchers at the National Eye Institute (NEI) announced they had identified, isolated, and characterized…

On Jul. 29, 2021, BOTOX(R) Allergan, an AbbVie company, announced that the U.S. Food and Drug Administration (FDA)…

On Jan. 7, 2021, BioNTech announced publication of preclinical data on its novel mRNA vaccine approach against autoimmune…

On Mar. 28, 2017, Genentech drug Ocrevus (ocrelizumab) was approved by the U.S. Food and Drug Administration (FDA)…
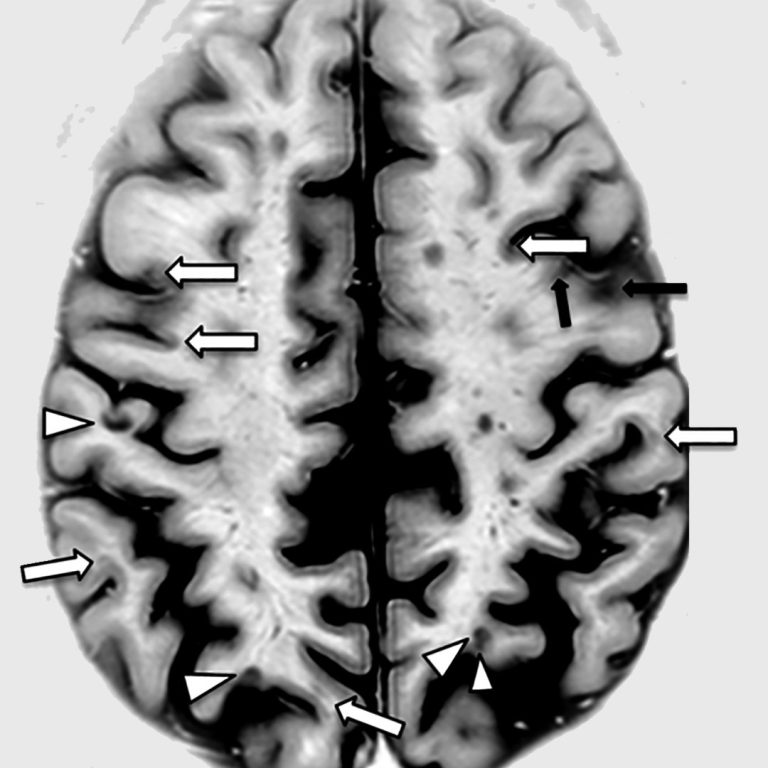
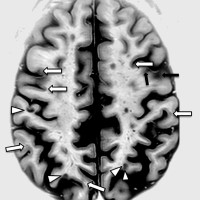
On Jun. 1, 2016, scientists at the University of British Columbia and Vancouver Coastal Health announced proof that…

On Apr. 21, 2011, Governor Mary Fallin dedicated the Oklahoma Medical Research Foundation’s (OMRF) new 186,000 s.f. research…

On Mar. 15, 2011, the Oklahoma Medical Research Foundation’s Multiple Sclerosis Center of Excellence, led by Dr. Gabriel…

On Feb. 16, 2016, Sanofi-aventis announced it would acquired Genzyme for $74 a share in cash or $21.1…
In 2008, University of California, San Francisco (UCSF) researchers discovered that B cells orchestrate the inflammation of myelin…
In 1998, some research caused concern that hepatitis B vaccination might be linked with multiple sclerosis (MS), a…

On Jul. 23, 1993, the U.S. Food and Drug Administration (FDA) approved Bayer’s Betaseron, the first of several…

On Jul. 23, 1993, Chiron and Berlex Laboratories announced that the U.S. Food and Drug Administration (FDA) had…

On Jun. 16, 1981, Dr. Thomas Waldmann with the National Cancer Institute (NCI), part of the National Institutes…