Mexico reports 53% increase in flesh-eating screwworm cases since July
On Aug. 27, 2025, Mexico has recorded 5,086 cases of flesh-eating screwworm in animals as of August 17,…

On Aug. 27, 2025, Mexico has recorded 5,086 cases of flesh-eating screwworm in animals as of August 17,…

On Jun. 30, 2025, the U.S. Department of Agriculture (USDA) announced risk-based port re-openings for cattle, bison, and…
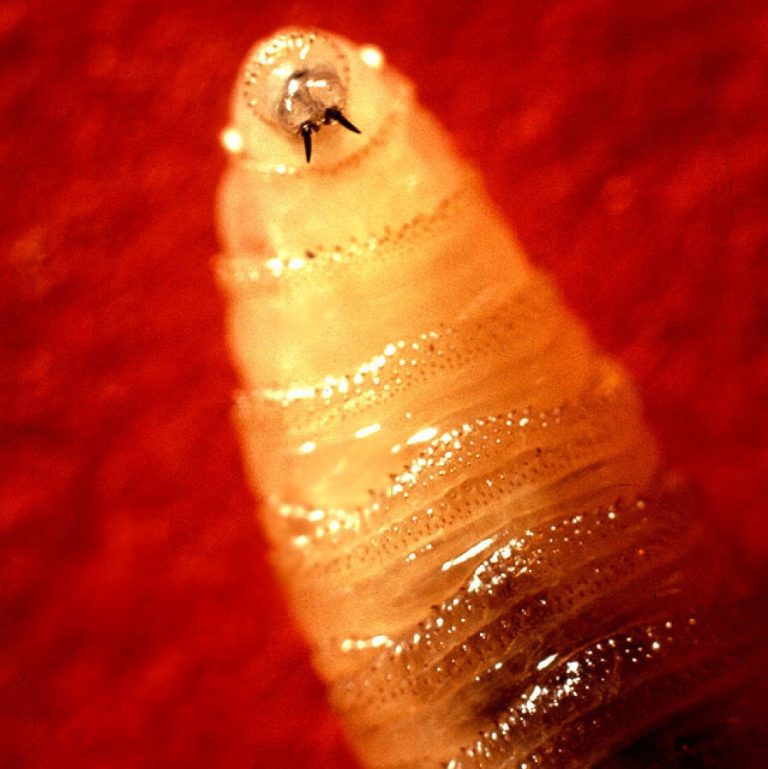
On Jun. 18, 2025, the U.S. Department of Agriculture announced it has launched an $8.5 million sterile New…

On Jun. 9, 2025, the Colorado Department of Public Health and Environment, the Arapahoe County Public Health Department,…

On Apr. 7, 2025, the Colorado Department of Public Health and Environment issued a Health Alert Network (HAN)…

On Apr. 4, 2025, a 3-year-old girl from the western state of Durango is Mexico’s first confirmed human…

On Apr. 2, 2025, the Colorado Department of Public Health and Environment issued a Health Alert Network (HAN)…
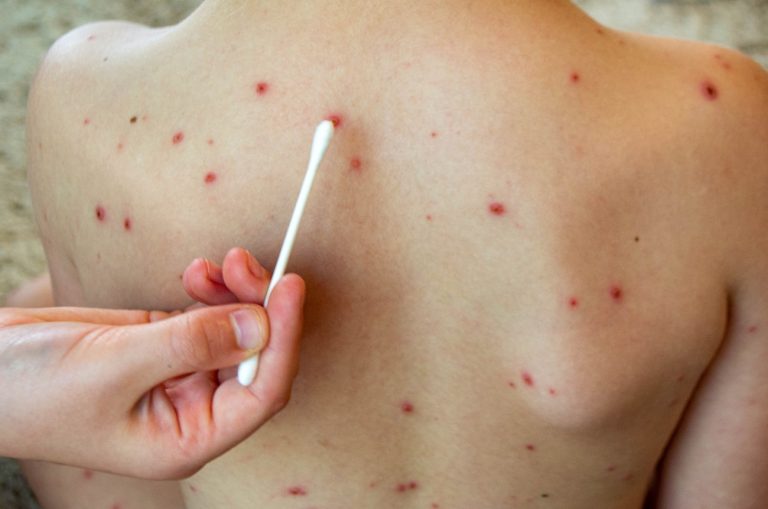
On Mar. 31, 2025, the Colorado Department of Public Health and Environment announced an unvaccinated adult in Colorado…

On Dec. 30, 2024, Texas Parks and Wildlife Department (TPWD) asked hunters and other outdoor enthusiasts in South…

In Dec. 20, 2024, the United States Trade Representative announced that the United States has prevailed in its…

On Dec. 13, 2024, the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service (APHIS) announced $165…

On Dec. 10, 2024, the Pan American Health Organization (PAHO) reported on three transmissible diseases affecting the Region…
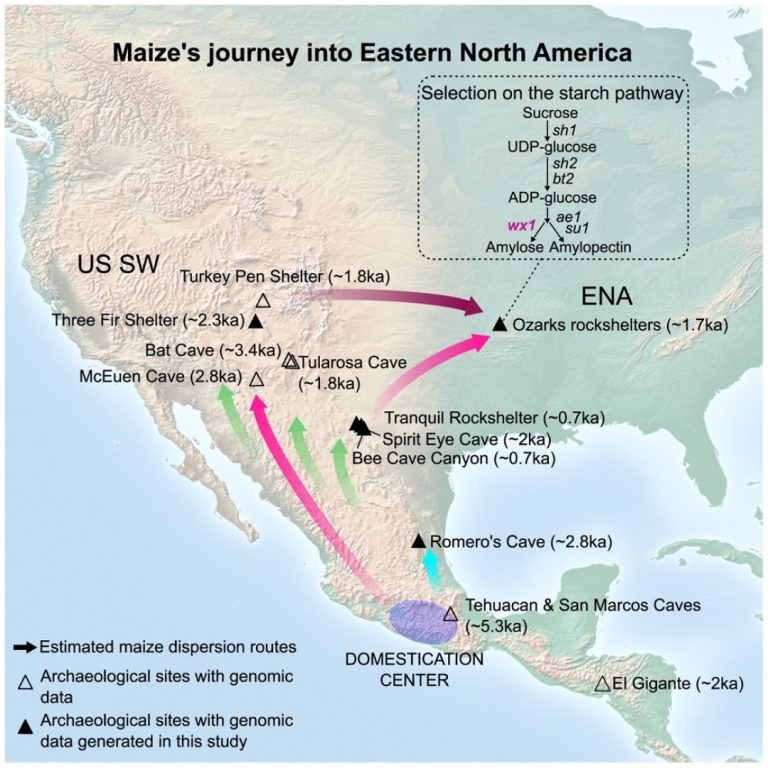
On Dec. 4, 2024, an international team of scientists led by the Center for Evolutionary Hologenomics, GLOBE Institute,…

On Nov. 26, 2024, the U.S. Department of Agriculture (USDA) announced it had temporarily paused imports of Mexican…

On Nov. 22, 2024, the Chief Veterinary Officer of Mexico notified the U.S. Department of Agriculture’s (USDA) Animal…

On Jun. 5, 2024, the World Health Organization (WHO) reported that a person with prior health complications who…
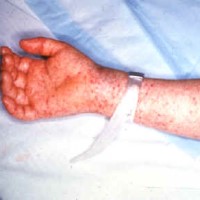
On Dec. 11, 2023, the U.S. Centers for Disease Control and Prevention (CDC) issued a Health Alert Network…

On Oct. 18, 2023, Novavax announced that the Medicines and Healthcare products Regulatory Agency (MHRA) in the United…

On Apr. 7, 2023, the U.S. National Park Service reported Highly Pathogenic Avian Influenza (HPAI) had been confirmed…

On Apr. 13, 2022, Novavax announced that Swissmedic had granted Novavax conditional marketing authorization (CMA) for Nuvaxovid COVID-19…

On Mar. 2, 2022, Sorrento Therapeutics announced that it had received clearance from the FDA for its investigational…

On Feb. 22, 2022, Moderna announced a distribution service agreement with Adium Pharma, a leading private Latin American…

On Feb. 14, 2022, Novavax announced its submission to Swissmedic, the Swiss Agency for Therapeutic Products, for conditional…

On Jan. 31, 2022, Novavax announced that it had submitted a request to the U.S Food and Drug…
On Dec. 27, 2021, Sorrento Therapeutics announced that initial testing of COVISTIX on recombinant N proteins demonstrated its…

On Dec. 13, 2021, Novavax announced that it had submitted a regulatory filing to the Ministry of Health…

On Nov. 24, 2021, Novavax announced its submission to the Singapore Health Sciences Authority for interim authorization of…

On Nov. 3, 2021, Inovio Pharma announced that it had received authorization from India’s Central Drugs Standard Control…

On Nov. 3, 2021, Novavax announced the company had filed for provisional approval of the vaccine to the…

On Oct. 11, 2021, Inovio Pharmaceuticals announced that it had received authorization from Colombia’s INVIMA, to conduct the…