Emergency vaccine response has cut infectious disease deaths by nearly 60% since 2000
On Jul. 14, 2025, researchers at Burnet Institute, in collaboration with Gavi, the Vaccine Alliance, have provided the…

On Jul. 14, 2025, researchers at Burnet Institute, in collaboration with Gavi, the Vaccine Alliance, have provided the…

On Apr. 16, 2025, A federal panel of medical experts has recommended an expansion of RSV vaccinations for…

On Nov. 12, 2024, researchers from the University of California, San Francisco (UCSF) reported that their genomic test…
On Nov. 7, 2024, researchers reported that Implementation of routine immunization of U.S. adolescents with the quadrivalent (four-strain)…
On Oct. 23, 2024, Pfizer announced that the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee…

On Aug. 27, 2024, the New Hampshire Department of Health and Human Services (DHHS) identified an adult who…
On Oct. 20, 2023, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved PENBRAYA (meningococcal…
On Oct. 2, 2023, the World Health Organization (WHO) announced it had recommended a new vaccine, R21/Matrix-M, developed…

On Jun. 22, 2022, Merck announced that that the U.S. Food and Drug Administration (FDA) had approved an…
On Sept. 25, 2020, the Advisory Committee on Immunization Practices (ACIP) recommended routine vaccination with a quadrivalent meningococcal…
On Apr. 23, 2020, University of Oxford researchers announced they had begun testing a COVID-19 vaccine in human…

On Jan. 23, 2020, two independent meningitis B studies were published in the New England Journal of Medicine…
On May 19, 2017, the U.S. Centers for Disease Control and Prevention (CDC) published Immunization Practices Advisory Committee…
On Nov. 4, 2016, the U.S. Centers for Disease Control and Prevention’s (CDC) Immunization Practices Advisory Committee (ACIP)…

On Jan. 23, 2015, Novartis announced the US Food and Drug Administration (FDA) had granted accelerated approval of…

On Jun. 20, 2014, the U.S. Centers for Disease Control and Prevention (CDC) published Immunization Practices Advisory Committee…

On Aug. 1, 2013, Novartis announced the U.S. Food and Drug Administration (FDA) had approved Menveo (Meningococcal [Groups…
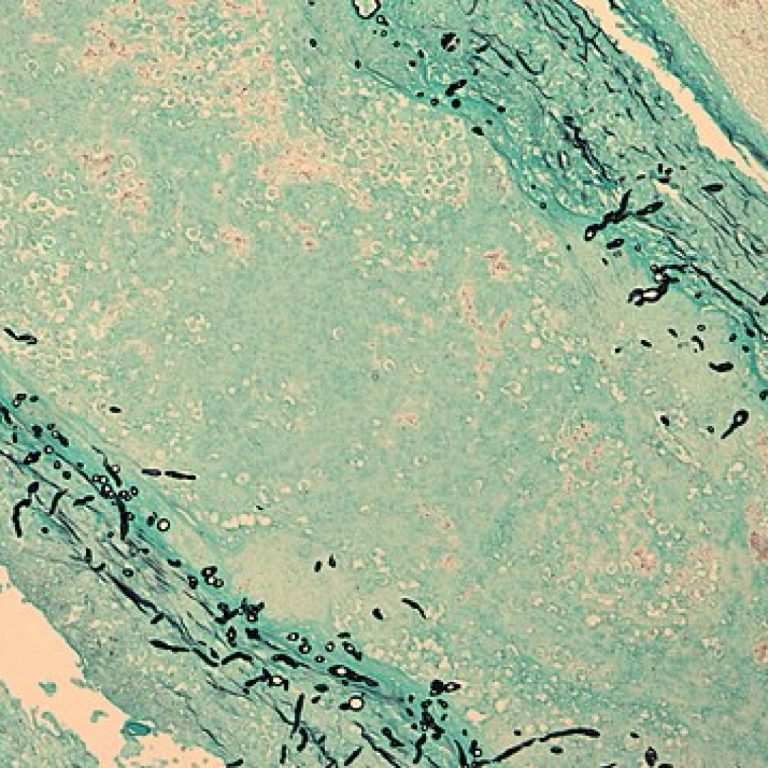
On Nov. 15, 2012, the U.S. Centers for Disease Control and Prevention (CDC) responded to the fungal meningitis…

On Jun. 24, 2012, the U.S. Food and Drug Administration (FDA) announced it had approved HibMenCY (Menhibrix, GlaxoSmithKline),…

On Apr. 22, 2011, The U.S. Food and Drug Administration (FDA) approved the use of Menactra in children…
On Feb. 19, 2010, the U.S. Food and Drug Administration (FDA) announced it had licensed a quadrivalent meningococcal…

On Oct. 17, 2007, the Food and Drug Administration (FDA) approved quadrivalent meningococcal conjugate vaccine (MCV4) (Menactra®, Sanofi…

On Aug 10, 2007, the U.S. Centers for Disease Control and Prevention (CDC) notified MMWR readers of revised…

On Apr. 22, 2005, Sanofi Pasteur announced the U.S. Food and Drug Administration (FDA) had granted licensure to…

On Jan. 14, 2005, the first meningococcal polysaccharide (Serogroups A, C, Y and W-135) diphtheria toxoid conjugate vaccine…

On May 29, 2001, the Bill & Melinda Gates Foundation announced today a global health grant of $70…

In 1999, a meningococcal group C conjugate vaccine was introduced into the routine schedule in the U.K. for…
On April 4, 1997, the Immunization Practices Advisory Committee (ACIP) recommended that the vaccine be used more extensively…

On Dec. 21, 1988, the conjugated Haemophilus influenzae type b vaccine (HibTITER by Wyeth-Lederle) was licensed. Prior to…
On Jan. 22, 1988, the Centers for Disease Control and Prevention’s (CDC) Immunization Practices Advisory Committee (ACIP) recommended…