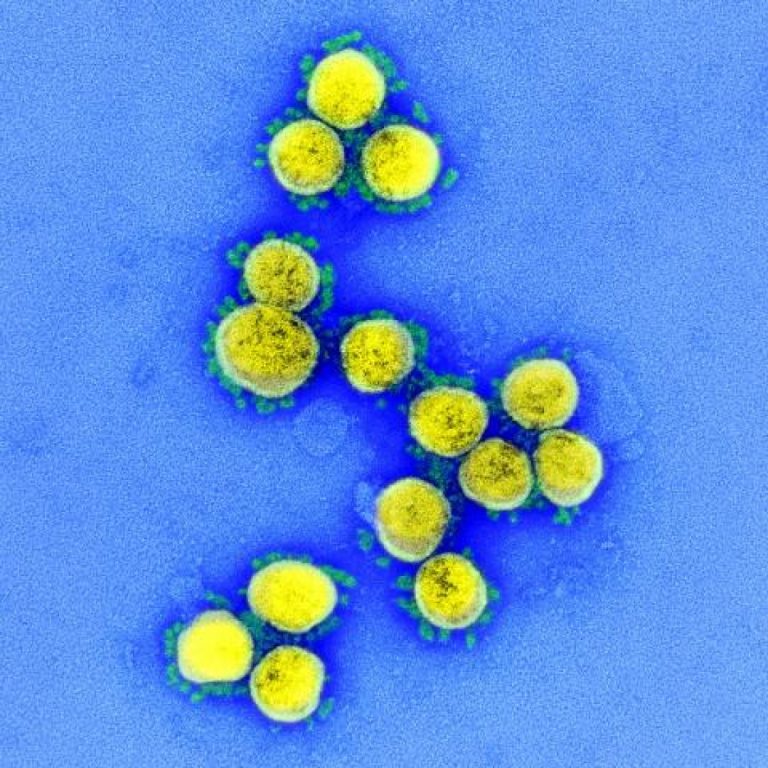
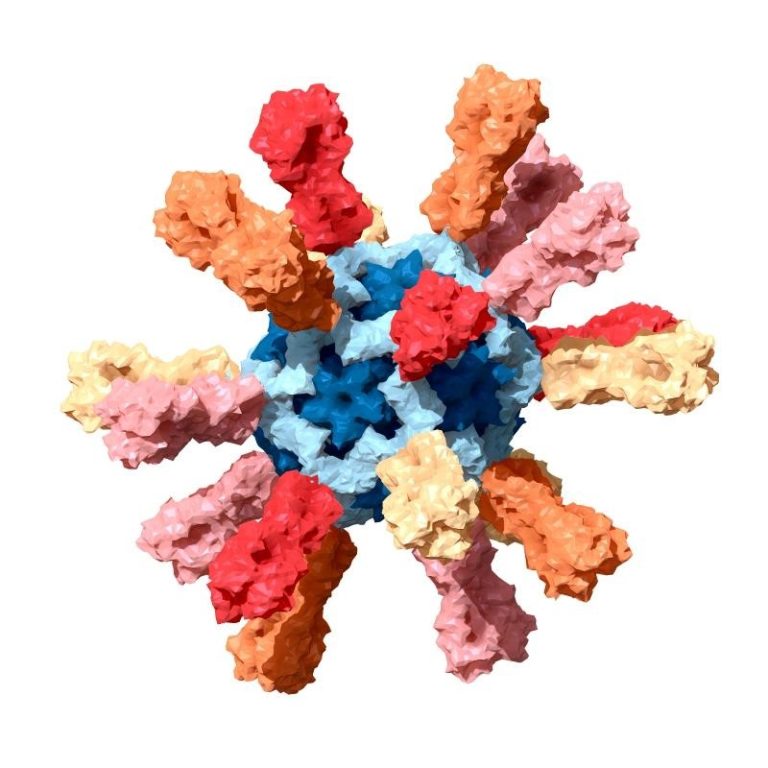
Takeda announced manufacture and provision of 150 million doses of Novavax COVID-19 vaccine candidate to the government of Japan
On Sept. 7, 2021, Takeda Pharmaceutical announced the Government of Japan’s Ministry of Health, Labour and Welfare purchase…