UT Southwestern scientists discover ancient viral DNA activates blood cell production during pregnancy
On Oct. 24, 2024, researchers from Children’s Medical Center Research Institute at UT Southwestern reported that ancient viral remnants…

On Oct. 24, 2024, researchers from Children’s Medical Center Research Institute at UT Southwestern reported that ancient viral remnants…
On May 2, 2023, the U.S. Preventive Services Task Force (USPSTF) published a final recommendation statement on screening…

On Mar. 17, 2022, Eiger BioPharmaceuticals announced that Peginterferon Lambda (Lambda) significantly reduced the risk of COVID-19-related hospitalizations…

On Oct. 18, 2021, a NIAID sponsored clinical trial found that treatment with the immunomodulator interferon beta-1a plus…

On Sept. 20, 2021, Eiger BioPharmaceuticals announced announced that Peginterferon Lambda (Lambda) will be added to the ongoing,…

On Sept. 14, 2021, the NIH announced that an international project called the COVID Human Genetic Effort has…

On May 3, 2021, Eiger BioPharmaceuticals announced announced that Peginterferon Lambda (Lambda) will be added to the ongoing,…

On Mar. 22, 2021, AIM ImmunoTech announced that the Institutional Review Board (IRB) of Roswell Park Comprehensive Cancer…

On Feb. 8, 2021, Eiger BioPharmaceuticals announced that the final results from the Phase 2 ILIAD (Interferon Lambda…

On Nov. 25, 2020, AIM ImmunoTech announced that Roswell Park Comprehensive Cancer Centerメs Phase 1/2a study evaluating the…

On Oct. 15, 2020, the World Health Organization (WHO) announced Interim that results from the Solidarity Therapeutics Trial,…

On Oct. 15, 2020, Eiger BioPharmaceuticals announced results of the ILIAD Study (Interferon Lambda for Immediate Antiviral Therapy…

On Sept. 28, 2020, Eiger BioPharmaceuticals announced results of an investigator sponsored study of Peginterferon Lambda-1a (Lambda) in…

On Sept. 16, 2020, AIM ImmunoTech announced that recruitment had begun in Roswell Park Comprehensive Cancer Center’s Phase…
On Aug. 27, 2020, Myriad RBM announced ultrasensitive immunoassays for viral pneumonia Including COVID-19 from our CLIA-certified laboratory….

On Aug. 5, 2020, a randomized, controlled clinical trial evaluating the safety and efficacy of a treatment regimen…

On May 14, 2020, AIM ImmunoTech announced the FDA authorized the first human trial assessing the safety and…

On Apr. 30, 2020, Eiger BioPharmaceuticals announced that the first patients have been dosed in a Phase 2…

On Apr. 21, 2020, Bayer Mississauga, Ontario announced a partnership with the Population Health Research Institute (PHRI) to…

On Dec. 3, 2002, Genentech drug Pegasys (peginterferon alfa-2a) was approved by the Food and Drug Administration (FDA)…
In 2001, the drug imatinib mesylate (Gleevec) was shown in a clinical trial to be effective against chronic…
On Jun. 1, 2000, scientists at the Oklahoma Medical Research Foundation (OMRF) announced the discovery of a new…

On Jul. 23, 1993, the U.S. Food and Drug Administration (FDA) approved Bayer’s Betaseron, the first of several…
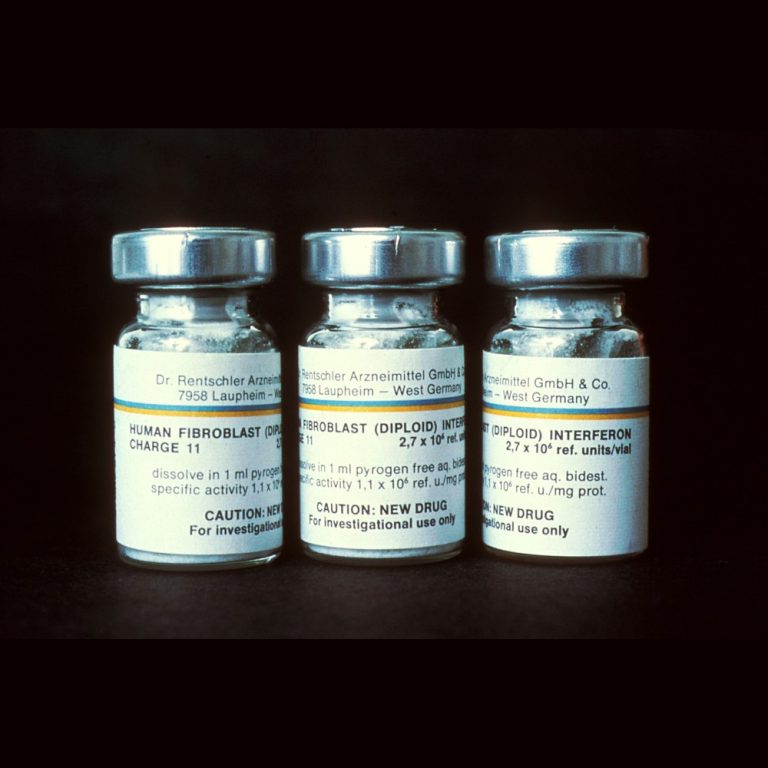
On Jun. 5, 1986, the U.S. Food and Drug Administration (FDA) approved Hoffmann La Roche’s Interferon initially for…
In May 1986, the vaccine Recombivax HB, which protects against hepatitis B infection, was approved for marketing in…
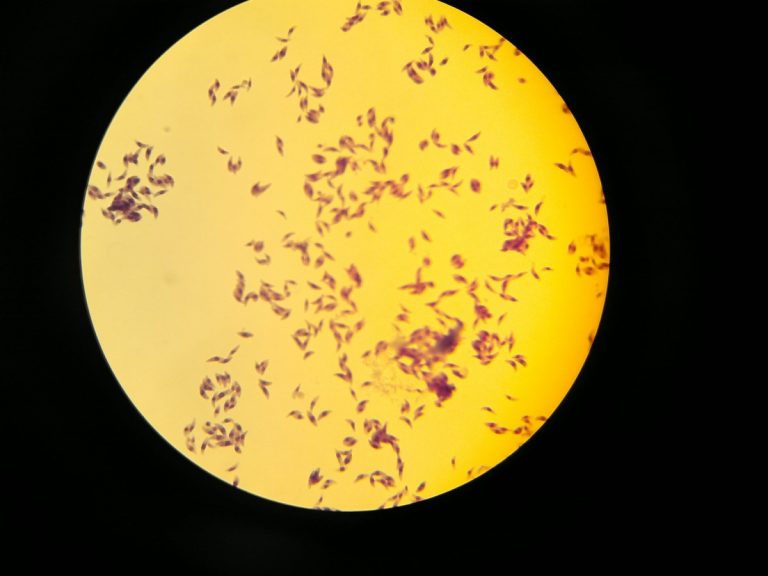
On Feb. 8, 1984, Elmer R. Pfefferkorn published his discovery that treatment of human fibroblasts with human recombinant…

On Nov. 9, 1978, the first human testing of a biological therapy was conducted (alpha-interferon). The Cancer Research…

In 1957, Alick Isaacs from Great Britain and Jean Lindenmann from Switzerland identified the interferons in 1957 and…