The FDA approved new therapy for triple negative breast cancer
On Apr. 22, 2020, the U.S. Food and Drug Administration (FDA) granted accelerated approval to Trodelvy (sacituzumab govitecan-hziy),…

On Apr. 22, 2020, the U.S. Food and Drug Administration (FDA) granted accelerated approval to Trodelvy (sacituzumab govitecan-hziy),…

On Jan. 21, 2019, researchers at Fred Hutchinson Cancer Research Center announced finding a way to essentially smother…

On Jul. 1, 2014, Genentech entered into an agreement to acquire Seragon Pharmaceuticals, a privately held biotechnology company…

On Sept. 14, 2007, the U.S. Food and Drug Administration (FDA) announced it had approved Eli Lilly’s osteoporosis…

On Dec. 7, 2005, results from several studies presented at the San Antonio Breast Cancer Symposium validated that…
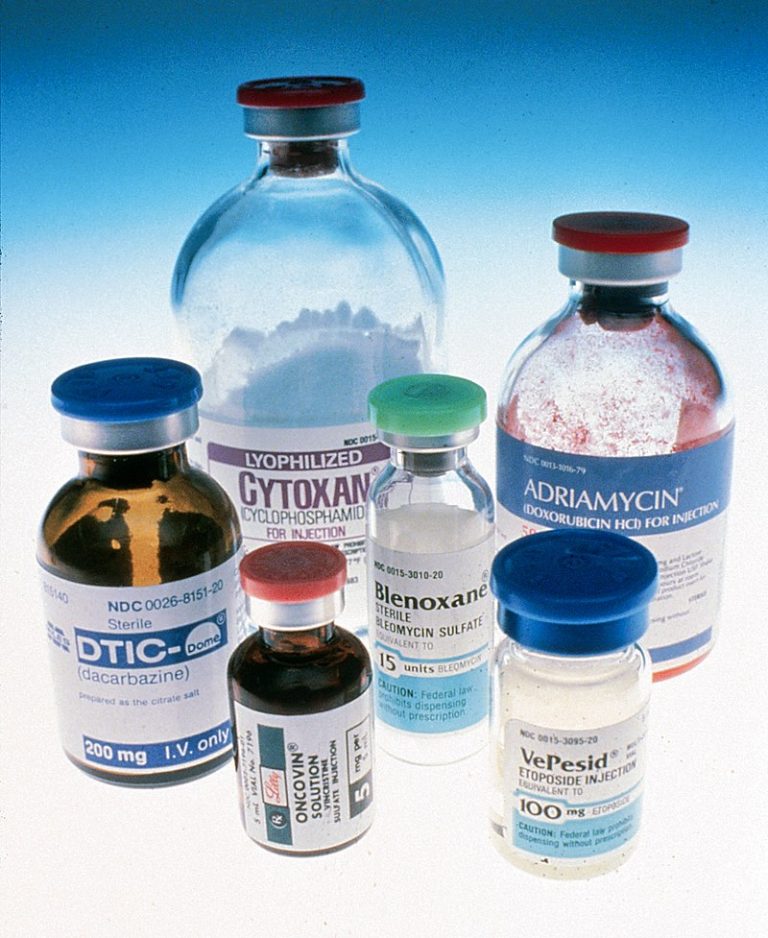
On Dec. 10, 2004, a National Cancer Institute (NCI) supported study determined that a new molecular test done…
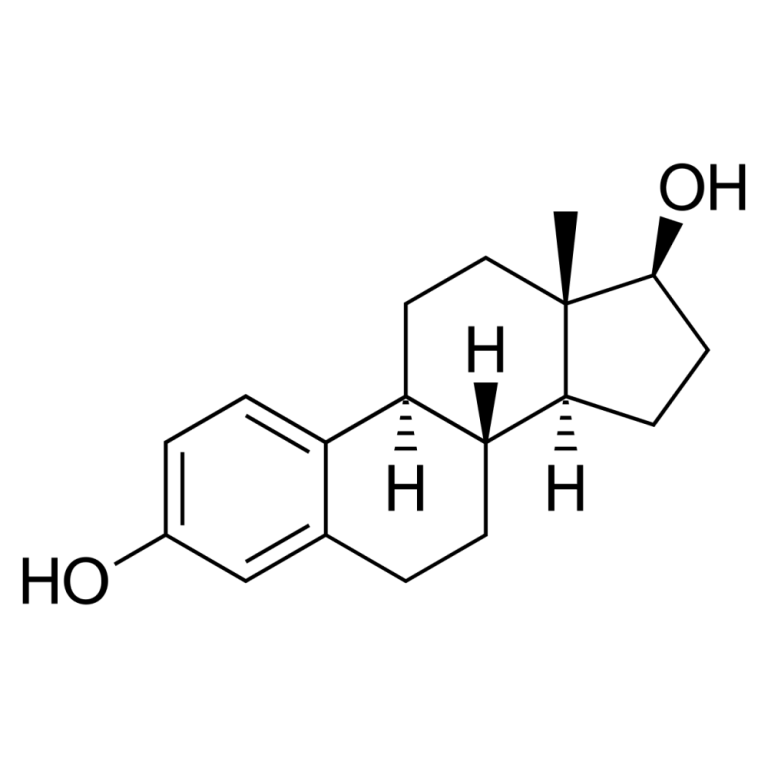
In 2004, data from the Women’s Health Initiative (WHI) study show that women who take estrogen in combination…

On Jul. 17, 2002, a National Cancer Institute funded trial showed that postmenopausal women who used estrogen replacement…
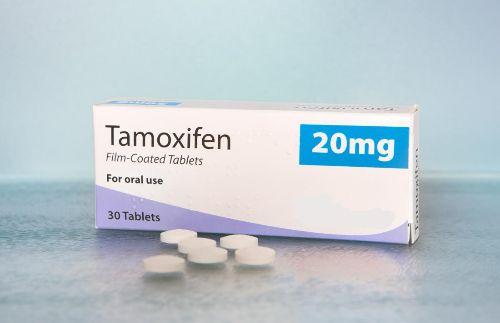
In 1999, Siteman Cancer Center investigators joined the STAR (Study of Tamoxifen and Raloxifene) trial for breast cancer…
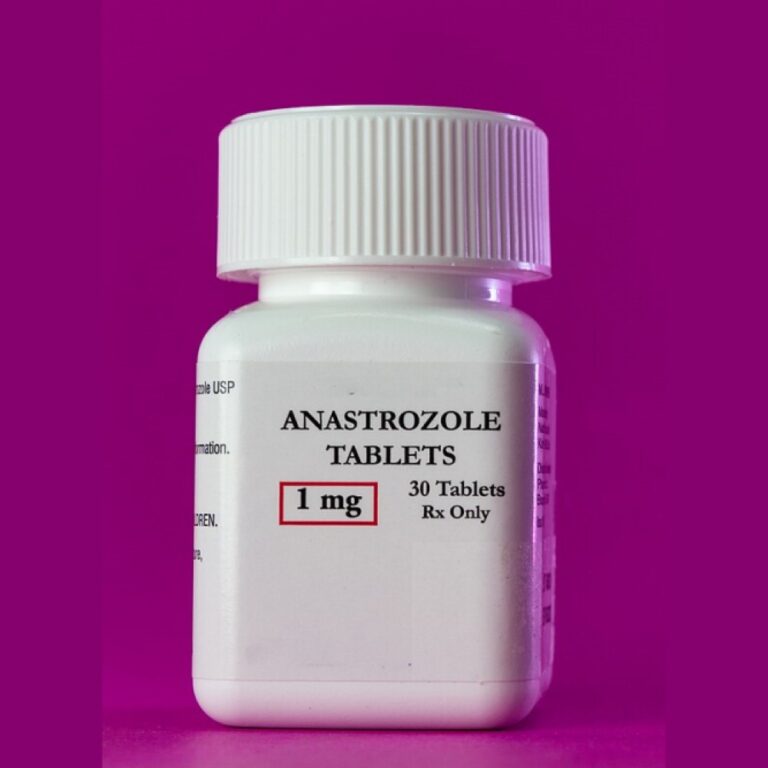
Dec. 27, 1995, the U.S. Food and Drug Administration (FDA) approved anastrozole (Arimidex) as a treatment for breast…

On Dec. 30, 1977, the U.S. Food and Drug Administration approved Tamoxifen, an anti-estrogen drug, for the treatment…
In 1973, Karmanos Cancer Institute researcher Herbert Soule, Ph.D. developed the MCF-7 human breast cancer cell line, which…

In 1941, Charles Huggins discovers that blocking male hormones (by removal of the testicles or administration of estrogens)…