Pediatric brain tumor treatment at the University of Alabama awarded FDA grant for first-in-human study
On Dec. 17, 2019, the University of Alabama at Birmingham Comprehensive Cancer Center (UAB) announced that Gregory Friedman,…

On Dec. 17, 2019, the University of Alabama at Birmingham Comprehensive Cancer Center (UAB) announced that Gregory Friedman,…
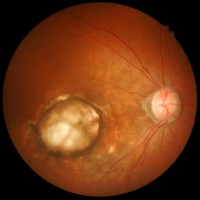
On Dec. 16, 2019, researchers at the National Eye Institute launched a clinical trial to test the safety…

On Dec. 11, 2019, Dennis Eggers became the first patient in a new clinical trial to receive a…
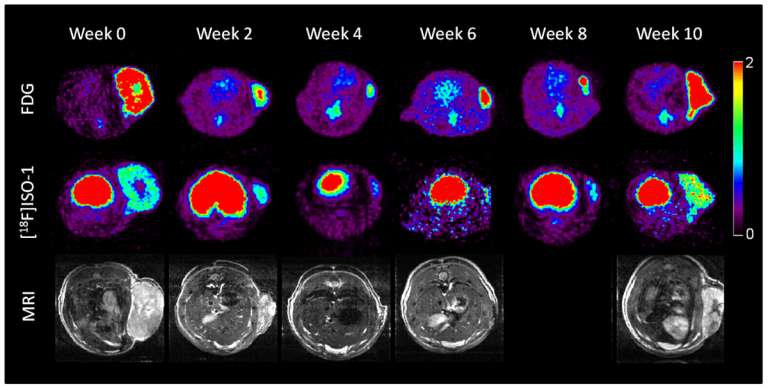
On Oct. 2, 2019, the Masonic Cancer Center and the University of Minnesota (UM) opened a new first-in-human…

On Aug. 1, 2019 the Siteman Cancer Center announced it was awarded a six-year, $7.8 million grant from…
On Jun. 20, 2019, CanSino Biologics announced that it had received the National Medical Products Administration’s (NMPA) permission…

On Dec. 20, 2018, the Karmanos Cancer Institute and Wayne State University were awarded the prestigious LAPS Grant…

On Dec. 5, 2018, Genomics England announced the 100,000 Genomes Project, done in partnership with National Health Service…

On Oct. 3, 2018, Scripps Research announced that Calibr scientitsts from Scripp’s drug development division had begun using…
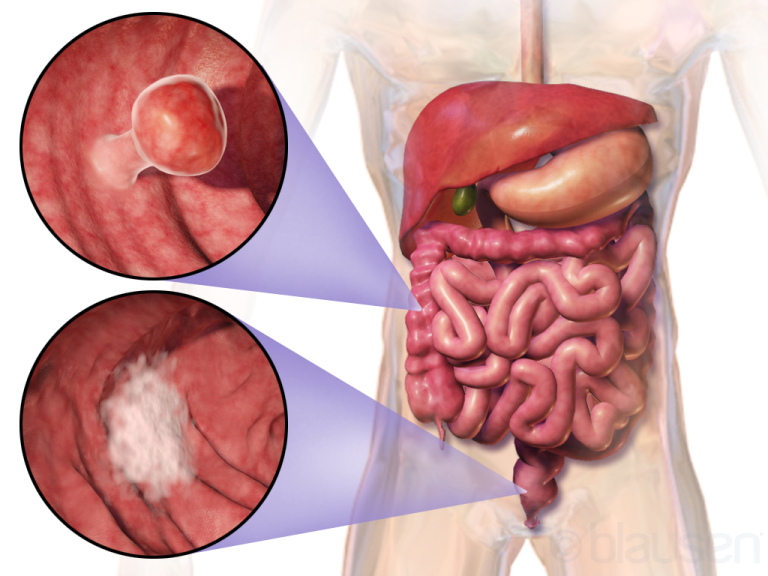
May 4, 2018, scientists from the National Cancer Institute (NCI) reported that an analysis of a prevention clinical…
On Nov. 28, 2017, surgeons at University of Washinton Medicine in Seattle announced they had performed the first…
On Oct. 23, 2017, GlaxoSmithKline announced the U.S. Food and Drug Administration (FDA) had approved Shingrix (Zoster Vaccine…

On Oct. 18, 2017, the Siteman Cancer Center became one of the first cancer centers nationwide to offer…

On Apr. 26, 2017, the University of Virginia Cancer Center was honored as one of just 69 National…

On Nov. 1, 2016, the Fred Hutchinson Cancer Research Center announced the opening of the Bezos Family Immunotherapy…

On Oct. 27, 2016, Roswell Park Cancer Institute announced it had received authorization from the U.S. Food and…

On Sept. 21, 2016, the National Institutes of Health (NIH) announced final rule requirements for submitting registration and…

On May 17, 2016, Bristol-Myers Squibb completed an expansion at its Devens, MA biologics facility designed to accelerate…

On Jul. 14, 2015, Benaroya Research Institute at Virginia Mason (BRI) announced that Carla J. Greenbaum, MD, had…

On May 5, 2015, Rutgers Cancer Institute of New Jersey was one of seven international sites to offer…

On Jan. 23, 2014, the National Institutes of Health (NIH) awarded the Benaroya Research Institute at Virginia Mason…

On Sept. 28, 2012, the Connecticut Challenge Survivorship Clinic opened at Yale Cancer Center providing — the first…

On Jan. 23, 2012, UC San Diego Health System announced it had received approval to acquire the Nevada…

On Jun. 24, 2012, Rock and Roll Hall of Fame inductee Alice Cooper helped raise $675,500 for the…

On May 25, 2011, the Roskamp Institute and an international research consortium led by Trinity College Dublin, were…

On Dec. 20, 2011, the U.S. Food and Drug Administration (FDA) announced approval of the first and only…
In 2011, the Pacific Northwest Diabetes Research Institute began clinical trials on antioxidant drug treatments on volunteers from…
On Feb. 19, 2010, the U.S. Food and Drug Administration (FDA) announced it had licensed a quadrivalent meningococcal…
On Dec. 3, 2009, the Thai HIV vaccine clinical trial results were published in the New England Journal…

On Sept. 16, 2009, Brian J. Druker, M.D.,whose research led to the development of Gleevec, the first targeted…