WHO announced preliminary results about dexamethasone use in treating critically ill COVID-19 patients
On Jun. 16, 2020, the World Health Organization (WHO) announced that initial clinical trial results from the United…

On Jun. 16, 2020, the World Health Organization (WHO) announced that initial clinical trial results from the United…

On Jun. 15, 2020, the U.S. Food and Drug Administration (FDA) revoked the emergency use authorization (EUA) that…

On Jun. 15, 2020, a team led by Scripps Research discovered antibodies in the blood of recovered COVID-19…

On Jun. 15, 2020, Immunic announced dosing of the first patients in its phase 2, CALVID-1 clinical trial…

On Jun. 13, 2020, Sinovac Biotech announced positive preliminary results of phase I/II clinical trial for the Companyメs…

On Jun. 11, 2020, Sinovac Biotech announced the signing of a clinical development collaboration agreement to advance the…

On Jun. 11, 2020, Regeneron announced initiation of the first clinical trial of REGN-COV2, its investigational dual antibody…

On Jun. 10, 2020, researchers funded by the National Institutes of Health (NIH) launched an effort to evaluate…

On Jun. 10, 2020, Johnson & Johnson announced that through its Janssen Pharmaceutical subsidiary it has accelerated the…

On Jun. 9, 2020, Immunic announced receipt of regulatory allowance from the FDA to initiate its phase 2,…

On Jun. 5, 2020, BioSig Technologies and its subsidiary, ViralClear Pharma, announced it had expanded its patient enrollment…

On Jun. 4, 2020, INOVIO and Seoul National University Hospital announced a partnership to start a Phase 1/2…

On Jun. 2, 2020, Agenus announced the FDA’s clearance of AgenTus’ IND application for an allogeneic iNKT therapy….

On Jun. 1, 2020, Tonix Pharmaceuticals announced an agreement whereby FUJIFILM Diosynth Biotechnologies will provide contract manufacturing and…

On Jun. 1, 2020, Altimmune announced the FDA had authorized the Company to proceed with a clinical trial…

On May 28, 2020, the Jenner Institute, University of Oxford, UK and Advent Srl ヨ an IRBM company,…

On May 27, 2020, Heat Biologics announced a collaboration with Waisman Biomanufacturing to establish a partnership for the…

On May 26, 2020, Novavax announced enrollment of the first participants in a Phase 1/2 clinical trial of…

On May 26, 2020, Montefiore Health System and Albert Einstein College of Medicine began the next stage of…

On May 21 2020, Amarin announced support for a clinical trial to investigate the effects of icosapent ethyl…

On May 20, 2020, BioSig Technologies and its subsidiary, ViralClear Pharmaceuticals, announced the closing of a $10.8 million…

On May 20, 2020, Diffusion Pharmaceuticals announced it had entered into an agreement with the Romanian National Institute…
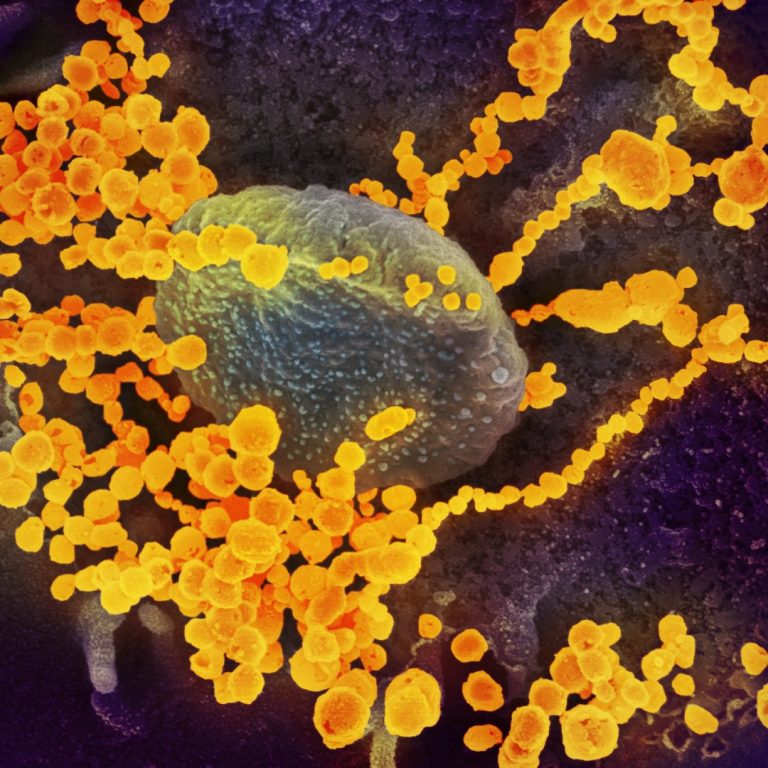
On May 19, 2020, CytoDyn announced it will be coordinating with the National Institution of Health of Mexico…
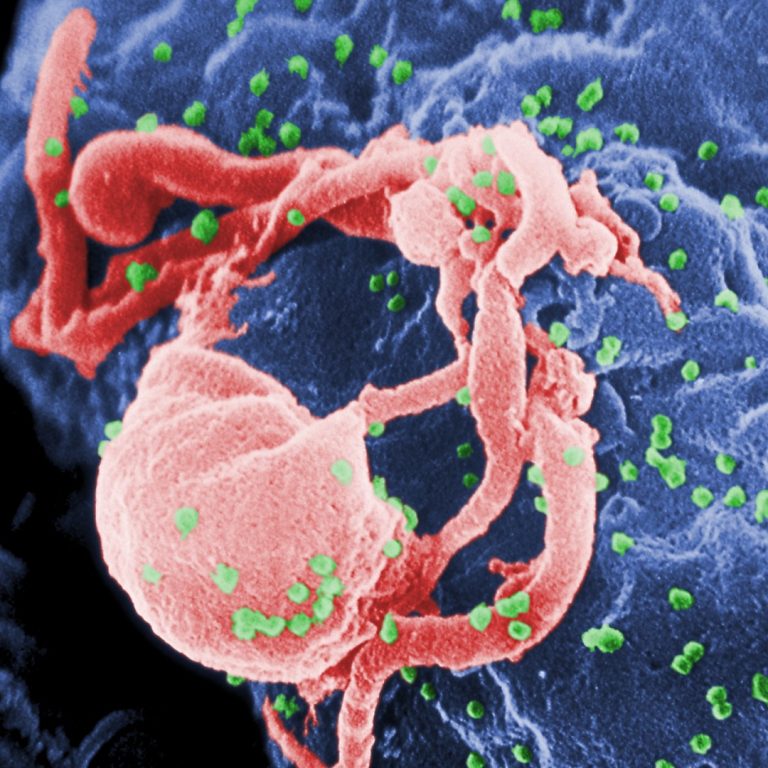
On May 18, 2020, an investigational long-acting form of the HIV drug cabotegravir injected once every 8 weeks…
On May 18, 2020, with $9 million from the COVID-19 Therapeutics Accelerator, an international group of physicians and…
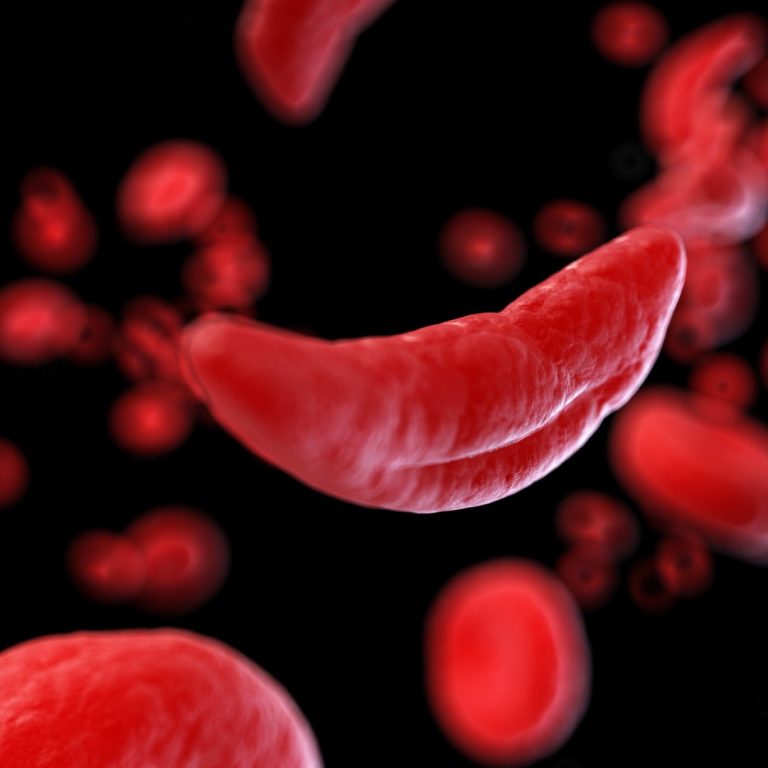
On May 15, 2020, the California Institute for Regenerative Medicine (CIRM) board approved new clinical trials for COVID-19…

On May 14, 2020, the National Institute of Allergy and Infectious Diseases (NIAID) announced that a clinical trial…

On May 13, 2020, Immunic announced it had received first regulatory approval from Germany’s BfArM to initiate a…

On May 12, 2020, the National Research Council of Canada (NRC) announced a collaboration with CanSino Biologics to…

On May 11, 2020, the FDA announced actions to accelerate the development of prevention and treatment options for…