China builds world’s most detailed human genome with ‘enormous’ implications for disease treatment
On Feb. 2, 2024, Chinese scientists announced they have assembled the world’s most detailed human genome yet, a…

On Feb. 2, 2024, Chinese scientists announced they have assembled the world’s most detailed human genome yet, a…

On Jan. 31, 2024, a U.S. Centers for Disease Control and Prevention (CDC) co-authored study published in Influenza…

On Jan. 27, 2024, the National Health Commission of the People’s Republic of China notified the World Health…

On Jan. 4, 2024, AstraZeneca Sanofi’s Beyfortus (nirsevimab), a long-acting monoclonal antibody, was approved in China for the…

On Nov. 23, 2023, the World Health Organization (WHO) announced that it was monitoring data from Chinese surveillance…
On Oct. 9, 2023, GlaxoSmithKline announced that it had reached an exclusive agreement with Chongqing Zhifei Biological Products…

On Apr. 14, 2023, the National Health Commission of the People’s Republic of China reported a confirmed case…

On Mar. 26,. 2023, Guangdong Centers for Disease Control in China reported an H3N8 infection that would become…

On Feb. 16, 2023, the Government of China announced it will plant less than 1% of its corn…

On Feb. 15, 2023, Researchers from Southwest University in China have constructed the entire chromosomal-scale genome assembly and…

On Jan. 31, 2023, a team of researchers led by the Innovation Center for Neurological Disorders and Department…

On Dec. 23, 2022, BioNTech and Fosun Pharmaceutical announced that they had received the certificates of registration as…
On Dec. 5, 2022, the Fred Hutchinson Cancer Center announced that according to a new meta-analysis women were…

On Oct. 27, 2022, Inovio Pharma announced that it has discontinued its internally funded efforts to develop INO-4800…
On Sept. 6, 2022, scientists in China announced that they had conducted the first comprehensive study of the…
On May 30, 2022, The Centre for Health Protection (CHP) of the Department of Health received notification from…

On May 19, 2022, WHO issued an emergency use listing (EUL) for CONVIDECIA, a vaccine manufactured by CanSino…
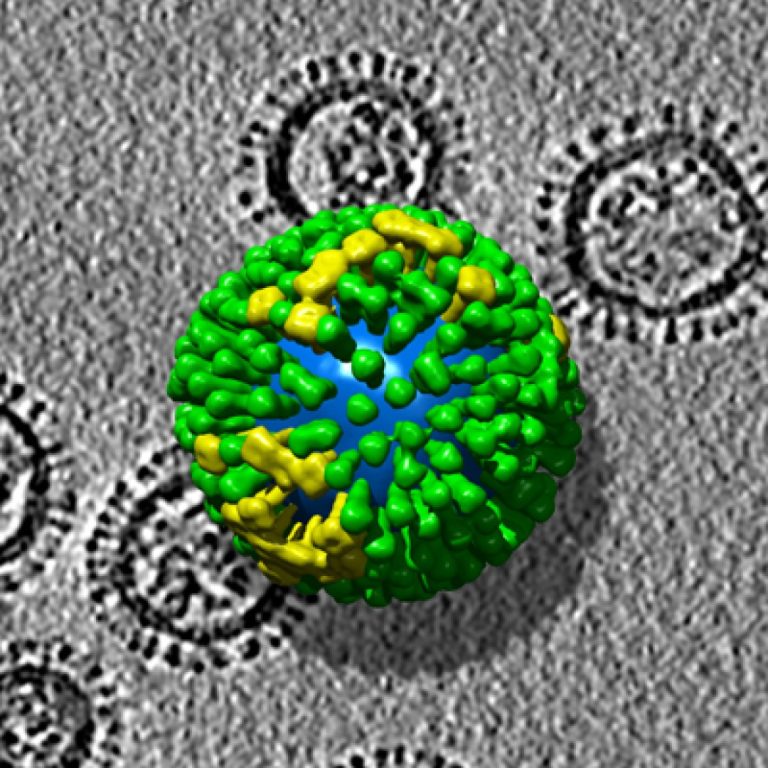
On Apr. 26, 2022, China’s National Health Commission reported a case of human infection with H3N8 avian influenza…
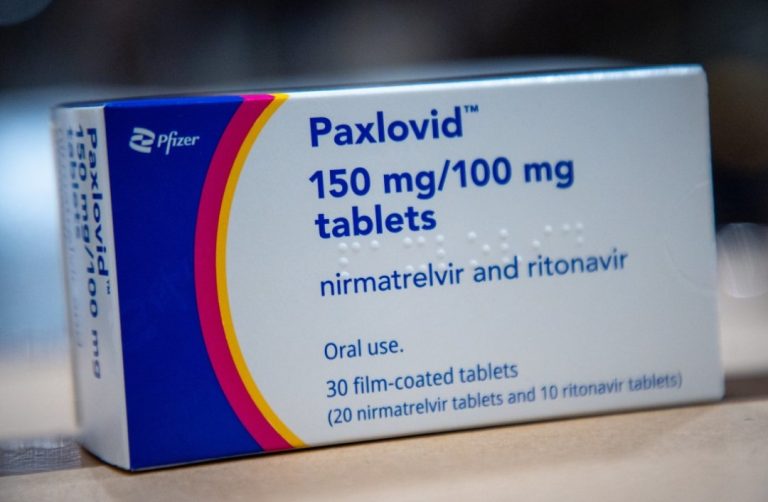
On Feb. 12, 2022, China’s medical products regulator announced that it had given conditional approval for Pfizer’s COVID-19…

On Oct. 21, 2021, Ligand Pharmaceuticals announced the signing of a collaboration agreement granting China Resources Double-Crane Pharmaceutical…

Around 8000 BCE, farmers in China, Egypt and Sumeria began using fermentation to produce cheese and wine. One…

On Aug. 9, 2021, Inovio Pharma announced that it had received regulatory allowance for two clinical trials investigating…

On Jul. 12, 2021, Gavi, the Vaccine Alliance, announced that it had signed advance purchase agreements with Sinopharm…

On Jun. 30, 2021, following a 70-year effort, China was awarded a malaria-free certification from the World Health…

On Jun. 1, 2021, WHO validated the Sinovac-CoronaVac COVID-19 vaccine for emergency use, giving countries, funders, procuring agencies…

On Jun. 1, 2021, the WHO announced it had validated the Sinovac-CoronaVac COVID-19 vaccine for emergency use, giving…

On May 7, 2021, the World Health Organization (WHO) listed the Sinopharm COVID-19 vaccine for emergency use, giving…

On Feb. 24, 2021, scientists at the University of Oxford reported that overall deaths did not increase in…

On Jan. 4, 2021, Inovio Pharmaceuticals and Advaccine Biopharmaceuticals announced that they had entered into a collaboration and…
On Dec. 21, 2020, the U.S. National Institutes of Health (NIH) announced a study published in the Proceedings…