Moderna announced supplying 12 million doses of Omicron-containing bivalent COVID-19 booster vaccines to Government of Canada
On Aug. 23, 2022, Moderna announced that the Government of Canada had exercised its option to purchase an…

On Aug. 23, 2022, Moderna announced that the Government of Canada had exercised its option to purchase an…

On Jul. 14, 2022, Moderna announced that Health Canada had approved the use of Moderna’s mRNA COVID-19 vaccine,…

On Jun. 23 2022, Novavax announced the filing of a Supplement to a New Drug Submission with Health…
On Jun. 14 2022, Oragenics announced the publication of an article co-authored by Oragenics and collaborators at Inspirevax…
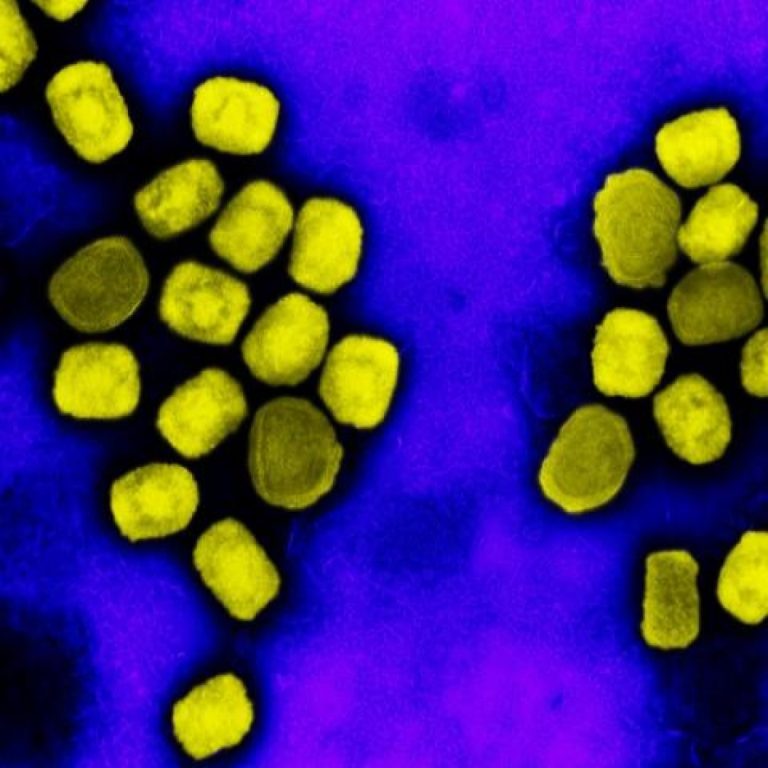
On May 21, 2022, the World Health Organization (WHO) reported that since May 13, 2022, cases of mpox…
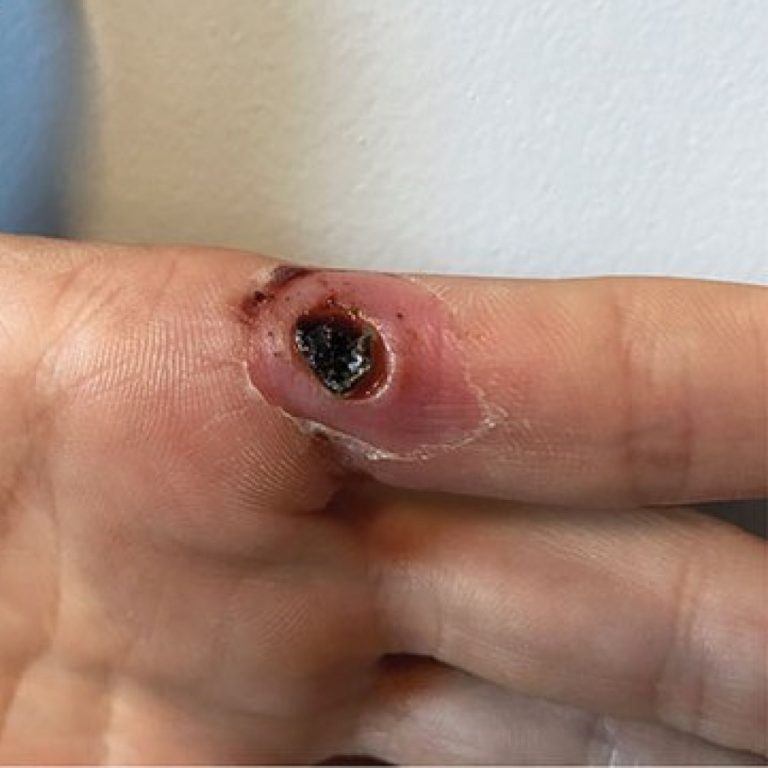
On May 18, 2022, scientists at the Centers for Disease Control and Prevention (CDC) announced that they were…

On Apr. 29, 2022, Moderna announced its plan to build a state-of-the-art mRNA vaccine manufacturing facility in Quebec…

On Apr. 12, 2022, the Province of Alberta poultry reported that farms had tested positive for highly pathogenic…

On Mar. 17, 2022, Moderna announced that Health Canada had approved the use of Moderna’s mRNA COVID-19 vaccine,…

On Mar. 2, 2022, the World Organization for Animal Health confirmed a bald eagle found dead in British…

On Feb. 24, 2022, Medicago and GlaxoSmithKline announced that Health Canada had granted approval for COVIFENZ, COVID-19 vaccine,…

On Feb. 17, 2022, Novavax announced that Health Canada had granted authorization for Nuvaxovid COVID-19 Vaccine (Recombinant protein,…

On Jan. 25, 2022, Cepheid announced that Health Canada had issued Cepheid a medical device license for Xpert…

On Jan. 6, 2022, two new studies published in the journal Current Biology showed that environmental DNA (eDNA)…

On Dec. 20, 2021, Oragenics announced it had extended a licensing and collaboration agreement with the National Research…
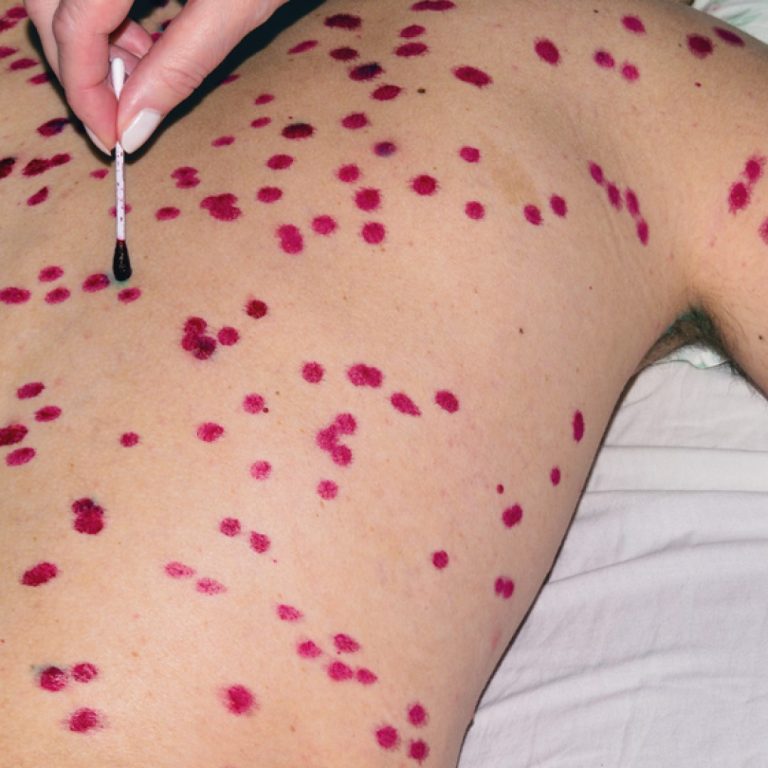
On Dec. 1, 2021, SIGA Technologies announced that Health Canada had approved oral TPOXX (tecovirimat) as an extraordinary…

On Nov. 15, 2021, Moderna confirmed that Health Canada had authorized the use of a booster dose of…

On Nov. 14, 2021, the Calgary city council voted to reinstate fluoridation in Calgary’s waters. The count was…

On Nov. 1, 2021, Novavax announced the completion of its rolling submission to Health Canada for authorization of…

On Oct. 6, 2021, Washington University School of Medicine announced the start of a pediatric COVID-19 vaccine clinical…

On Sept. 16, 2021, Moderna announced Health Canada had approved the New Drug Submission (NDS-CV) for SPIKEVAX (elasomeran…

On Sept. 8, 2021, National Resilience announced an agreement to manufacture mRNA for the Moderna COVID-19 vaccine. Under…

On Aug. 16, 2021, Moderna announced a revised supply agreement with the Government of Canada for up to…

On Aug. 10, 2021, Moderna announced a Memorandum of Understanding (MoU) with the government of Canada to build…

On Jul. 27, 2021, Oragenics announced it had entered into a licensing agreement with the National Research Council…

On Jun. 3, 2021, Resverlogix announced a partnership to support planned commercialization of apabetalone in the United States,…

On Jun. 1, 2021, AquaBounty Technologies announced the regulatory approval of the Company’s GE Atlantic salmon by Brazil’s…

On May 24, 2021, Quidel announced that its Sofia’ SARS Antigen FIA was the first rapid antigen test…

On May 18, 2021, GlaxoSmithKline and Medicago reported positive interim Phase 2 clinical trial safety and immunogenicity data…

On May 18, 2021, National Resilience announced that the Government of Canada will invest CAD 199.2 million ($163.8…