Some antibodies outmaneuver germs from sticking to cells
On Jan. 6, 2026, a study led by University of Washington School of Medicine researchers uncovered several new…

On Jan. 6, 2026, a study led by University of Washington School of Medicine researchers uncovered several new…

On Dec. 18, 2025, the National Aeronautics and Space Administration (NASA) announced it has opened the International Space…

On Sept. 16, 2025, Eli Lilly announced that it plans to build a $5 billion manufacturing facility just…
On Jan. 17, 2025, scientists from Scripps Research Institute reported finding that some HIV vaccination can lead to…

On Nov. 15, 2024, a team led by researchers from Sweden’s Karolinska Institutet reported study results that showed…
On Nov. 6, 2024, researchers from Emory University, in collaboration with scientists at Gladstone Institutes and University of…

On Jun. 14, 2024 the Centers for Disease Control and Prevention (CDC) reported data from this study suggested…
On Apr. 12, 2024, Oregon Health Sciences University (OHSU) announced research that shows using live SARS-CoV-2 virus revealed…
On Mar. 1, 2024, researchers at the National Institutes of Health (NIH) announced they had identified antibodies targeting…

On Sept. 6, 2023, Moderna announced that clinical trial data from its research assay confirmed its updated COVID-19…
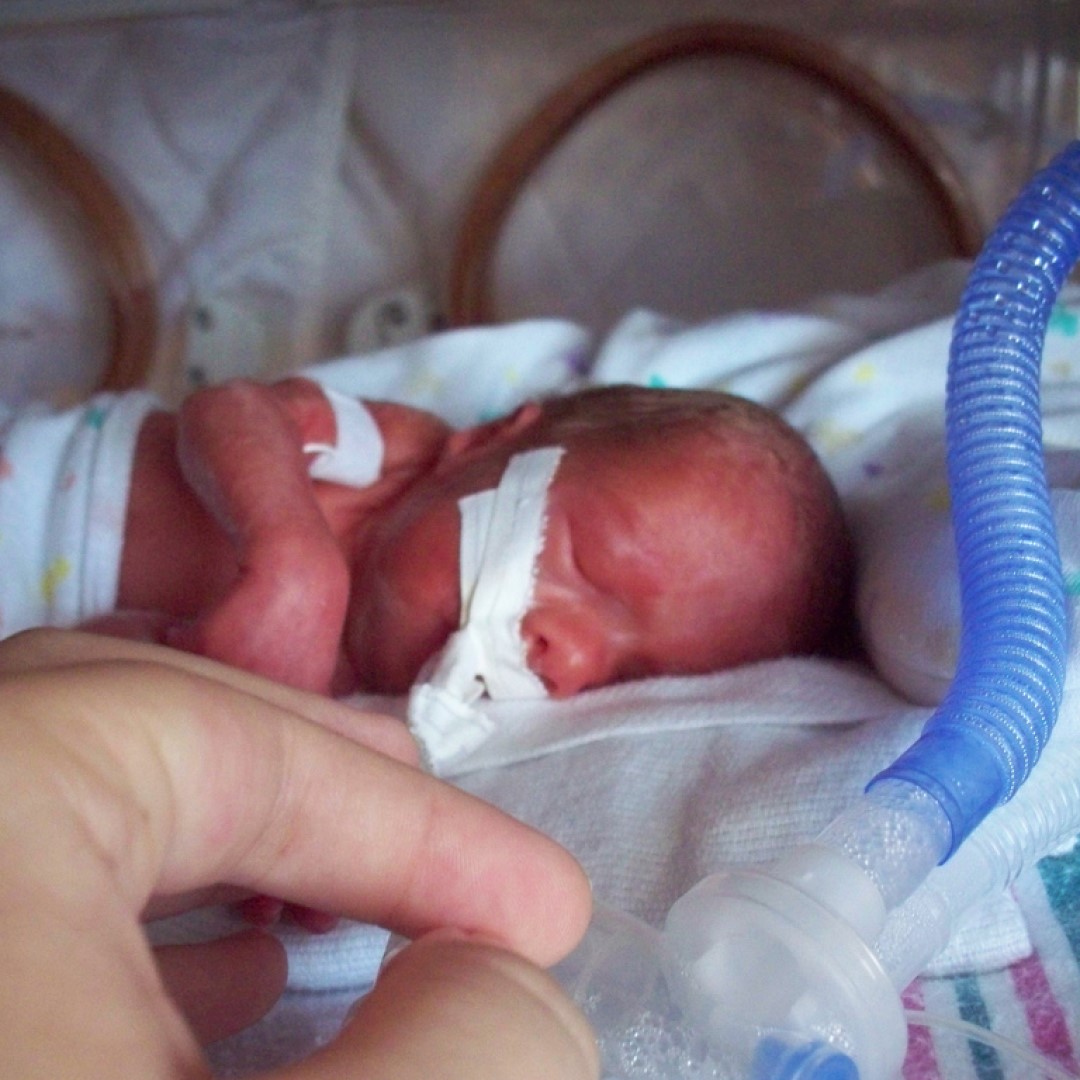
On Aug. 11, 2023, a study funded by the National Institute of Allergy and Infectious Diseases (NIAID) reported…

On Jul. 6, 2023, University of Illinois Urbana-Champaign researchers reported they can detect exposure to the SARS-CoV-2 virus…

On Jun. 29, 2023, the U.S. Food and Drug Administration approved Roctavian, an adeno-associated virus vector-based gene therapy…
On Apr. 27, 2023, a NIAID-led Phase 2 trial compared two Pfizer bivalent mRNA COVID-19 boosters found that…

On Mar. 24. 2023, antibiotics do not reduce the risk of dying in adults hospitalized with COVID-19, the…
On Aug. 24, 2022, BioMarin Pharmaceutical announced that the European Commission (EC) had granted conditional marketing authorization (CMA)…
On Jul. 19, 2022, the NIH announced that although COVID-19 booster vaccinations in adults elicit high levels of…

On Jul. 11, 2022, Moderna announced new clinical data on its bivalent Omicron (BA.1) booster candidate, mRNA-1273.214. One…

On Jul. 5, 2022, Tgen and Northern Arizona University announced that a research team found that the vaccine…

On Jul. 5, 2022, the National Institutes of Health released a study that described the immune response triggered…

On Apr. 7, 2022, Moderna and IAVI announced a collaboration to employ mRNA technology to meet the challenge…

On Feb. 17, 2022, after studying blood samples from 244 patients hospitalized for COVID-19, a group of researchers,…

On Jan. 28, 2022, the WHO announced that COVID-19 information had reached 1,292,209 people through Viamoメs 3-2-1 Platform…
On Jan. 27, 2022, NIAID announced that a clinical trial found that the combination of remdesivir plus a…

On Jan. 25, 2022, researchers at the University of Missouri announced they had identified the highly prevalent, specific…
On Jan. 24, 2022, Pfizer and BioNTech announced the publication of new results from two laboratory studies demonstrating…

On Jan. 20, 2022, scientists from the National Institute of Allergy and Infectious Diseases (NIAID) announced that they…

On Dec. 8, 2021, Pfizer and BioNTech announced results from an initial laboratory study demonstrating that serum antibodies…

On Dec. 7, 2021, Rockefeller University scientists announced a study had demonstrated the therapeutic potential of an unusual…

On Nov. 23, 2021, the World Health Organization’s COVID-19 Technology Access Pool and the Medicines Patent Pool finalized…