NIH launched clinical trial of universal influenza vaccine candidate
On Jun. 1, 2021, the National Institutes of Health (NIH) announced the first-in-human, Phase 1 trial assessing the…
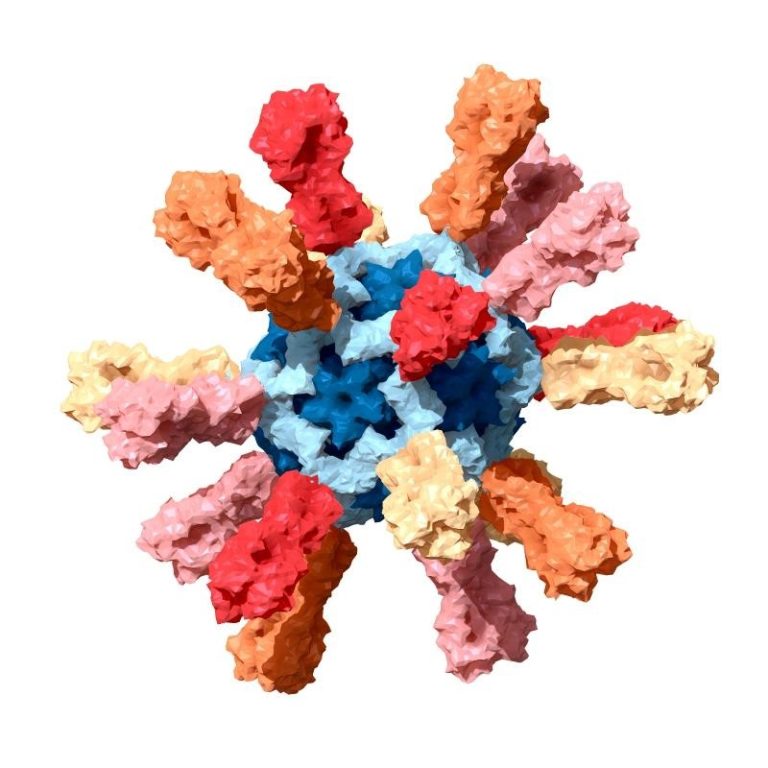
On Jun. 1, 2021, the National Institutes of Health (NIH) announced the first-in-human, Phase 1 trial assessing the…

On Jun. 1, 2021, the National Institutes of Health announced that it had started a Phase 1/2 clinical…
On May 26, 2021, National Institutes of Health (NIH) scientists reported that the immune system’s attempt to eliminate…
On Apr. 29, 2021, researchers at the University of British Columbia (UBC) announced a study published in Cell…

On Apr. 23, 2021, the National Institute of Allergy and Infectious Diseases (NIAID) announced that it had awarded…

On Apr. 21, 2021, the NIH announced that a Phase 2/3 trial to evaluate a new fully-human polyclonal…

On Apr. 15, 2021, the National Institute of Allergy and Infectious Diseases (NIAID) announced it had had established…

On Apr. 15, 2021, The National Institute of Allergy and Infectious Diseases (NIAID) announced that the fourth iteration…

On Apr. 13, 2021, a clinical trial testing the safety and efficacy of an investigational monoclonal antibody for…
On Apr. 12, 2021, Roche confirmed positive results from the phase III REGN-COV 2069 trial assessing the ability…

On Mar. 31, 2021, the National Institutes of Health (NIH) announced that an investigational vaccine designed to protect…
On Mar. 30, 2021, the National Institute of Allergy and Infectious Diseases (NIAID) announced that researchers had analyzed…
On Apr. 1, 2021, Kaiser Permanente Washington Health Research Institute (KPWHRI) announced that an investigational vaccine designed to…

On Feb. 23, 2021, the NIH announced that a randomized, placebo-controlled Phase 1 clinical trial of two monoclonal…

On Feb. 8, 2021, the NIH announced that an international randomized, controlled Phase 3 clinical trial had begun…
On Jan. 26, 2021, NIH researchers announced that an investigational anti-HIV antibody delivered intravenously once every eight weeks…

On Jan. 26, 2021, Regeneron announced positive initial results from an ongoing Phase 3 clinical trial evaluating REGEN-COV…
On Jan. 21, 2021, NIH researchers announced that after people recover from infection with a virus, the immune…

On Dec. 21, 2020, Aimmune Therapeutics announced that the European Commission (EC) had approved PALFORZIA [defatted powder of…

On Dec. 16, 2020, National Institute of Allergy and Infectious Diseases reported that an observational study had launched…

On Dec. 3, 2020, a new study by researchers at the University of British Columbia and BC Children’s…

On Nov. 25, 2020, the National Institute of Allergy and Infectious Diseases (NIAID), one of the National Institutes…

On Oct. 22, 2020, Moderna announced that it had completed enrollment of 30,000 participants for the Phase 3…
On Oct. 8, 2020, a clinical trial to test the safety, tolerability and efficacy of a combination treatment…
On Oct. 8, 2020, a clinical trial to test the safety, tolerability and efficacy of a combination treatment…
On Sept. 29, 2020, the National Institute of Allergy and Infectious Diseases announced that a phase 1 trial…

On Sept. 29, 2020, a phase 1 trial of an investigational mRNA vaccine to prevent SARS-CoV-2 infection was…
On Sept. 14, 2020, Eli Lilly and Incyte announced initial data from the Adaptive COVID-19 Treatment Trial (ACTT-2)…

On Sept. 1, 2020, HDT Bio received a notice of award from the National Institute of Allergy and…

On Aug. 31, 2020, BioCryst Pharmaceuticals announced that the National Institute of Allergy and Infectious Diseases (NIAID) had…