WHO to identify pathogens that could cause future outbreaks and pandemics
On Nov. 21, 2022, the WHO is launching a global scientific process to update the list of priority…

On Nov. 21, 2022, the WHO is launching a global scientific process to update the list of priority…
On Nov. 1, 2022, the Medicines Control Authority of Zimbabwe announced that it had approved the use of…

On Oct. 18, 2022, global leaders confirmed US$ 2.6 billion in funding toward the Global Polio Eradication Initiative’s…
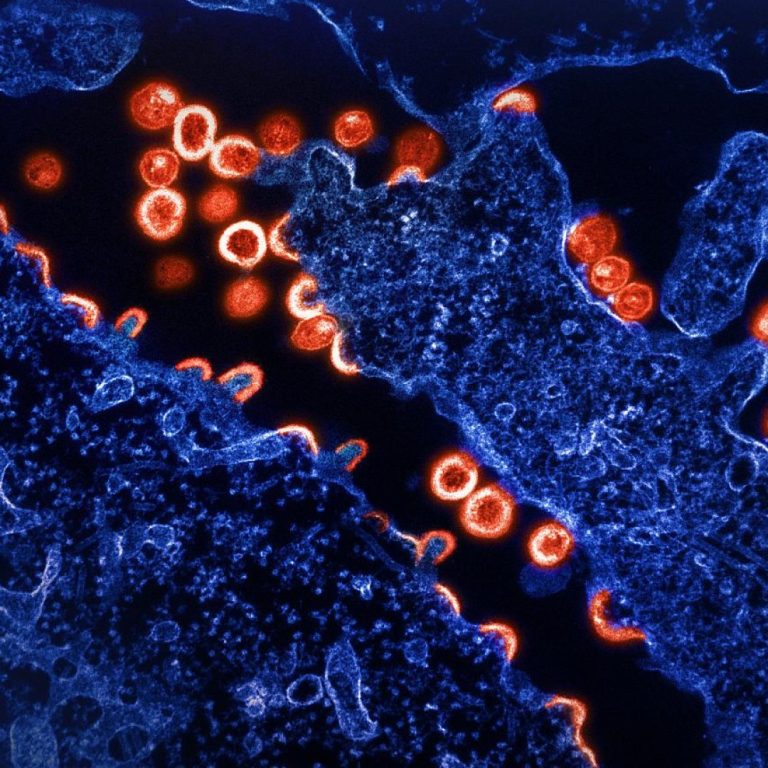
On Sept. 29, 2022, the World Health Organization announced that it had identified the spread of Anopheles stephensi…
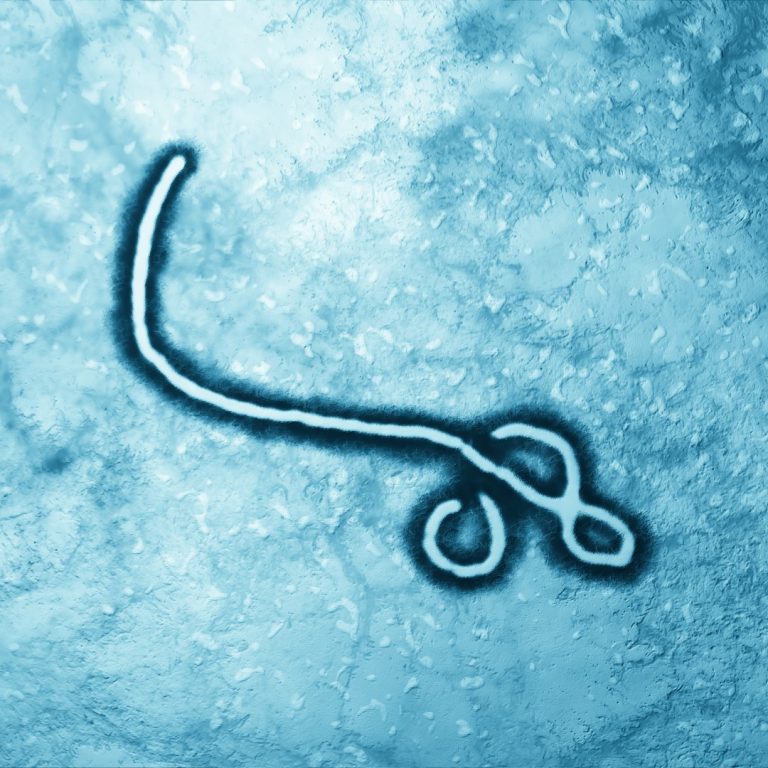
On Sept. 17, 2022, the Democratic Republic of the Congo declared the end of an Ebola outbreak that…

On Sept. 2, 2022, Novavax announced that the World Health Organization (WHO) had approved a variation to allow…
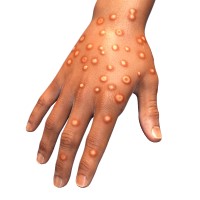
On Aug. 12, 2022, a group of global experts convened by WHO agreed on new names for monkeypox…
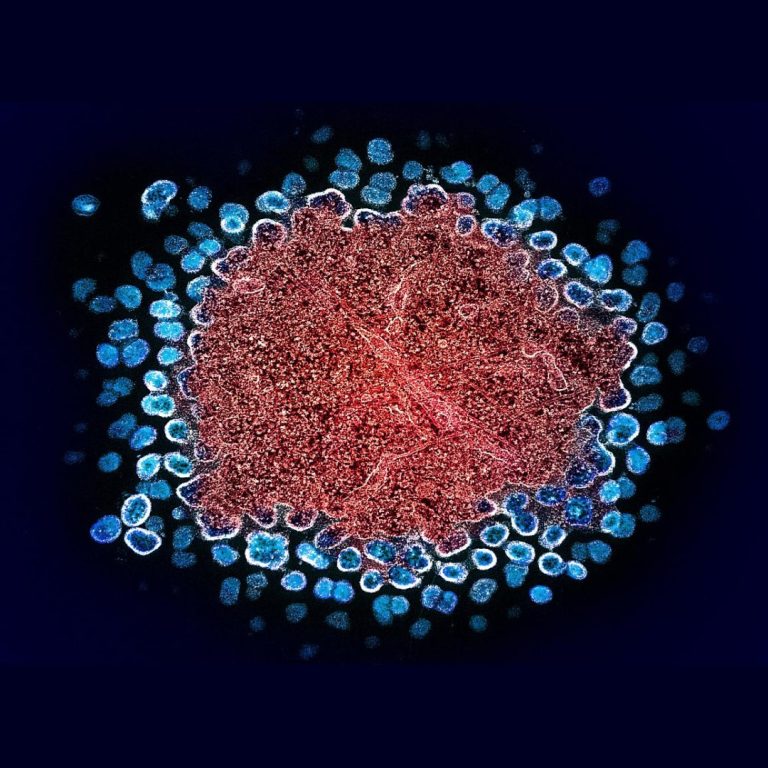
On Jul. 28, 2022, the World Health Organization (WHO) released new guidelines for the use of long-acting injectable…

On Jul. 15, 2022, The largest sustained decline in childhood vaccinations in approximately 30 years has been recorded…
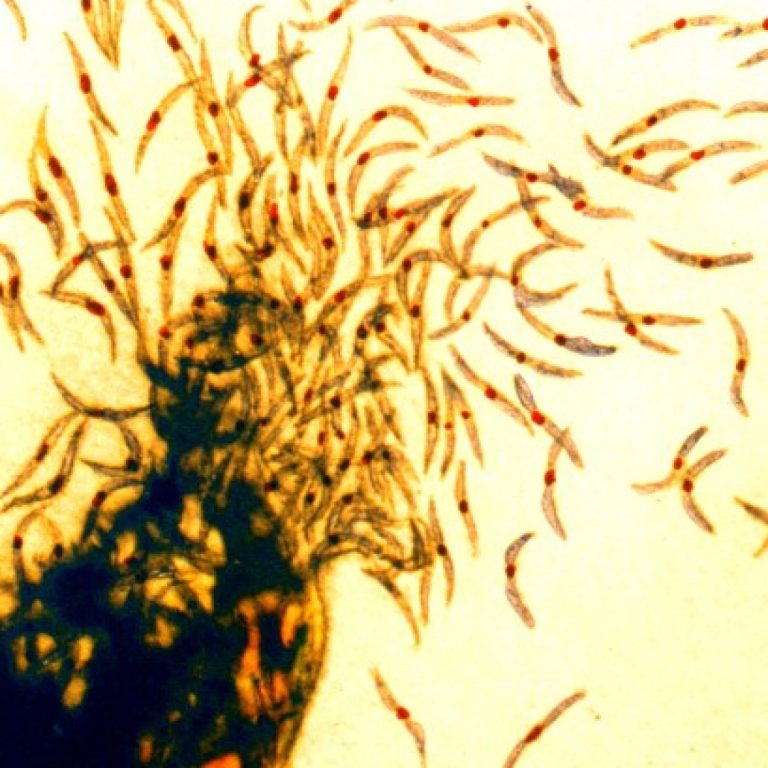
On Jul. 7, 2022, Ghana announced the preliminary finding of two cases of Marburg virus disease and if…
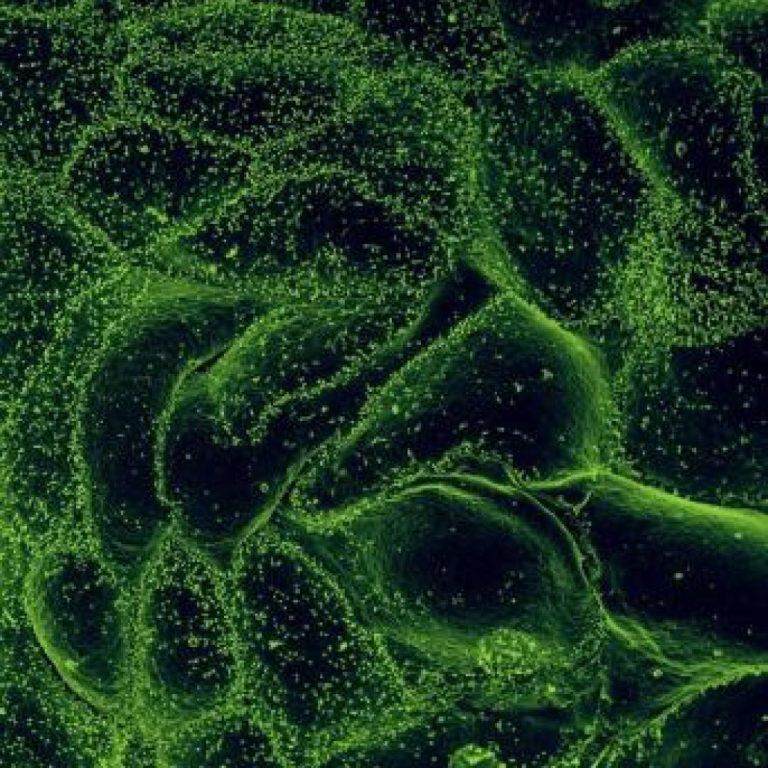
On Jul. 4, 2022, the Democratic Republic of the Congo declared the end of the Ebola outbreak that…
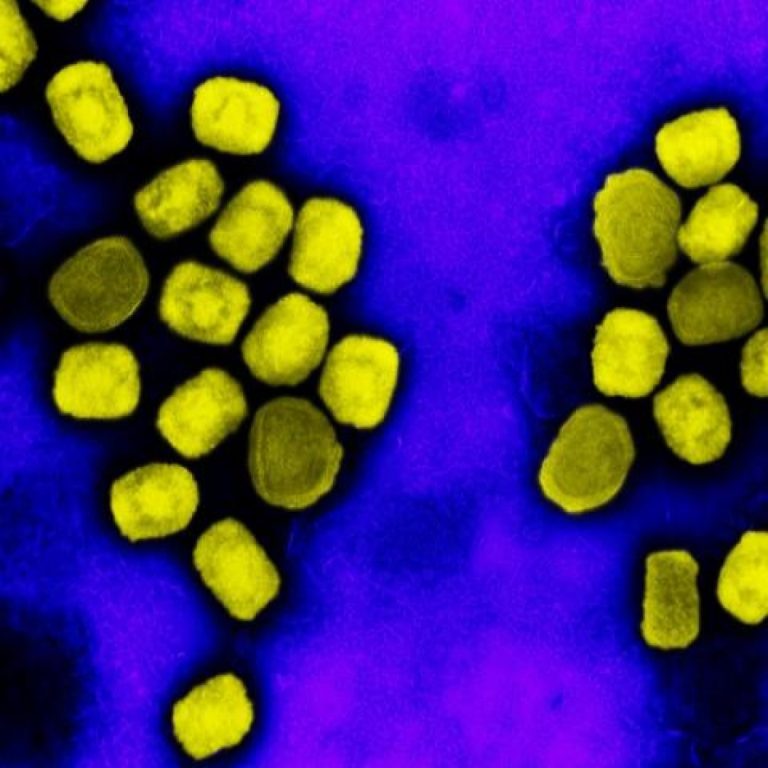
On May 21, 2022, the World Health Organization (WHO) reported that since May 13, 2022, cases of mpox…

On May 21, 2022, the WHO announced that since May 13, 2022, cases of monkeypox had been reported…

On May 19, 2022, WHO issued an emergency use listing (EUL) for CONVIDECIA, a vaccine manufactured by CanSino…

On May 12, 2022, WHOメs COVID-19 Technology Access Pool and the Medicines Patent Pool finalized a licensing agreement…
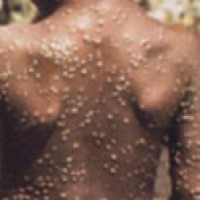
On May 7, 2022, WHO was informed of a confirmed case of monkeypox in an individual who travelled…

On May 5, 2022, the World Health Organization (WHO) reported estimates that show the full death toll associated…
On Apr. 28, 2022, the Ministry of Health of the Democratic Republic of the Congo declared an outbreak…

On Apr. 22, 2022, the World Health Organization (WHO) made a strong recommendation for nirmatrelvir and ritonavir, sold…

On Mar. 25, 2022, the WHO and the Government of India signed an agreement to establish the WHO…
On Mar. 18, 2022, the World Health Organization (WHO) announced that Malawi had launched the first round of…

On Mar. 4, 2022, the WHO announced it had published an updated position paper on the RTS,S/AS01 (RTS,S)…

On Mar. 3, 2022, the World Health Organization (WHO) and the Medicines Patent Pool (MPP) jointly welcomed the announcement…

On Mar. 2, 2022, Roche announced an initial donation of essential medicines to Ukraine. Roche is working diligently…

On Feb. 22, 2022, Sorrento Therapeutics announced that additional preclinical results demonstrate broad spectrum COVISHIELD (STI-9167) neutralizing activity…

On Feb. 18, 2022, the WHO announced the first six countries that will receive the technology needed to…

On Feb. 11, 2022, Roche announced that Actemraᆴ/RoActemraᆴ (tocilizumab) intravenous (IV) had been granted World Health Organization (WHO)…

On Feb. 11, 2022, the WHO announced the addition of tocilizumab, a monoclonal antibody, to its list of…

On Jan. 28, 2022, the WHO announced that COVID-19 information had reached 1,292,209 people through Viamoメs 3-2-1 Platform…

On Jan. 16, 2022, the WHO announced that it had shipped 1.1 million COVID-19 vaccines to Rwanda on…