Avian Influenza A(H5N1) outbreak reported in Cambodia
On Sep. 2, 2024, the World Health Organization (WHO) confirmed that a laboratory-confirmed case of human infection with…

On Sep. 2, 2024, the World Health Organization (WHO) confirmed that a laboratory-confirmed case of human infection with…
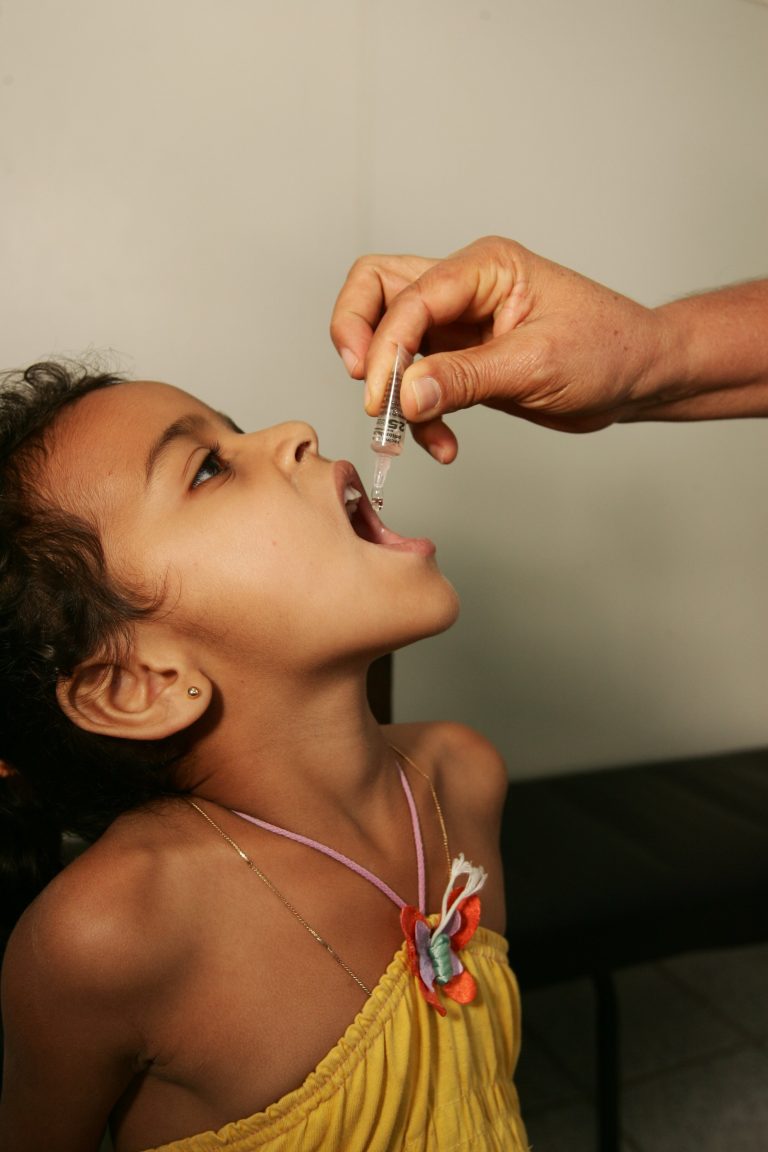
On Aug. 30, 2024, the United Nations (UN) announced that polio vaccination will begin for some 640,000 children…
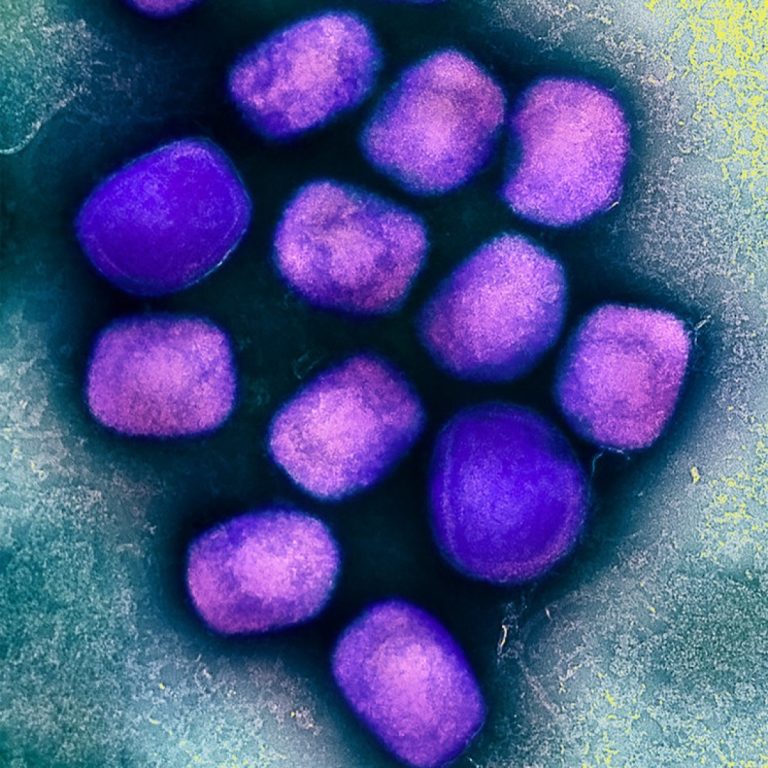
On Aug. 26, 2024, the World Health Organization (WHO) launched a global Strategic Preparedness and Response Plan to…

On Aug. 23, 2024, the World Health Organization announced that between early June and 15 August 2024, the…

On Aug. 23, 2024, the World Health Organization (WHO) reported an outbreak of Oropouche virus (OROV) in the…
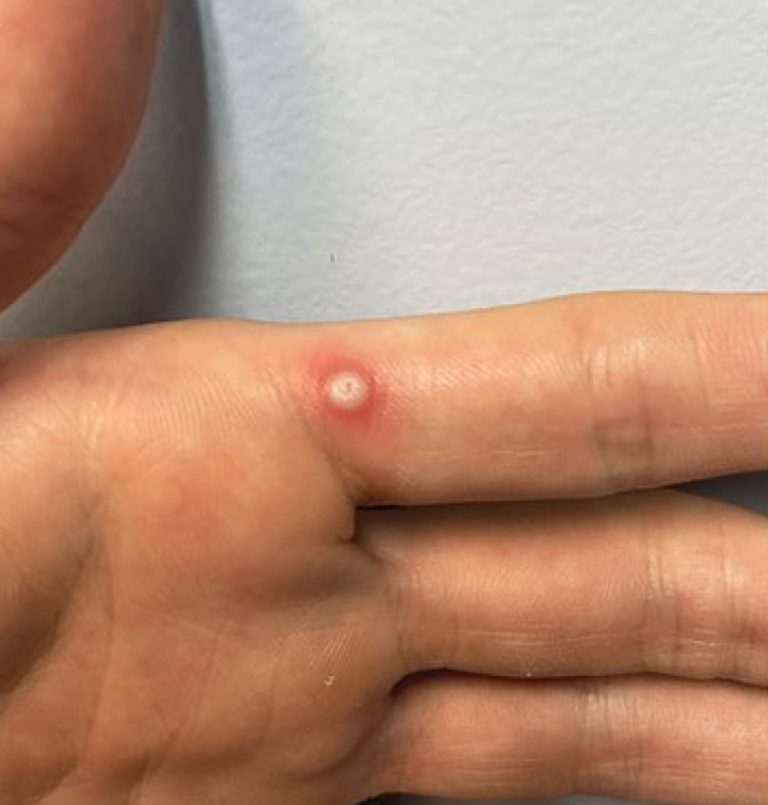
On Aug. 14, 2024, The World Health Organization declared that the upsurge of mpox in the Democratic Republic…

On Aug. 8, the World Health Organization (WHO) announced results of a study that showed the introduction of…

On Aug. 1, 2024, the Coalition for Epidemic Preparedness Innovations (CEPI) and the World Health Organization (WHO) called…

On Jul. 29, 2024, a new project that aims to accelerate the development and accessibility of human avian…
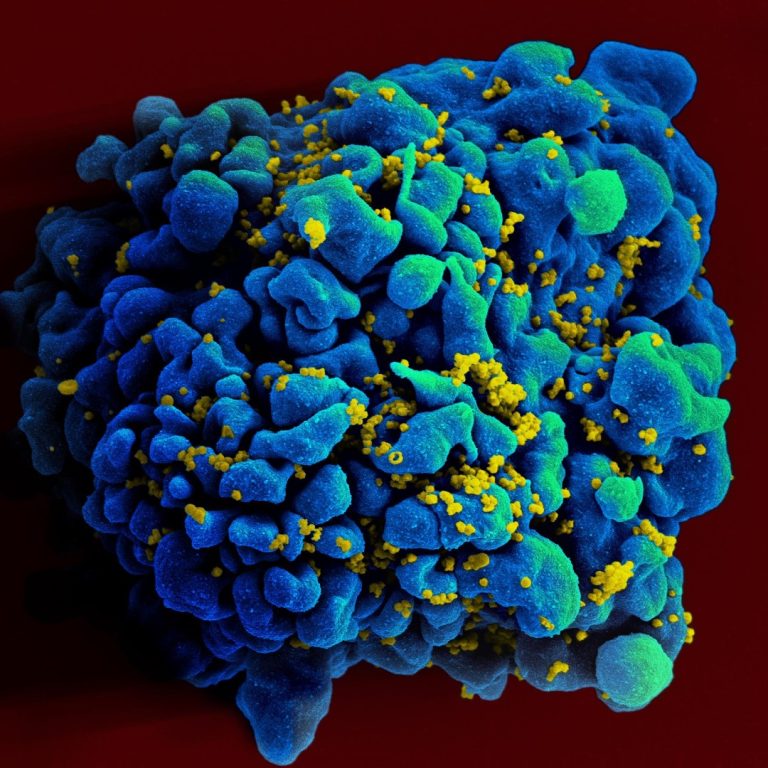
On Jul. 25, 2024, the World Health Organization (WHO) announced during the 25th International AIDS Conference, being held…

On Jul. 2, 2024, the World Health Organization (WHO) recommended a comprehensive set of tobacco cessation interventions, including…

On Jun. 26. 2024, the World Health Organization reported that new data show that nearly one third (31%)…

On Jun. 5, 2024, the World Health Organization (WHO) reported that a person with prior health complications who…

On May 30, 2024, the World Health Organization (WHO) reported that as of 30 April 2024, over 7.6…

On May 24, 2024, the World Health Organization (WHO) revealed that the COVID-19 pandemic reversed the trend of…
On May 22, 2024, the World Health Organization (WHO) announced that the first confirmed case of human infection…
On Mar. 27, 2024, the World Health Organization announced it had launched a network for coronaviruses, CoViNet, to…
On Feb. 21, 2024, the World Health Organization (WHO) reported that more than half the world’s countries will…
On Feb. 1, 2024, the World Health Organization (WHO) announced that new estimates on IARCメs Global Cancer Observatory…

On Jan. 27, 2024, the National Health Commission of the People’s Republic of China notified the World Health…

On Jan. 12, 2024, the World Health Organization (WHO) announced it had certified Cabo Verde as a malaria-free…

On Dec. 28, 2023, the International Health Regulations National Focal Point (IHR NFP) of Argentina notified the World…

On Dec. 21, 2023, the World Health Organization (WHO) announced that it had prequalified the R21/Matrix-M malaria vaccine…

On Dec. 15, 2023, In a pivotal move towards addressing one of the worldメs most underrecognized health challenges,…
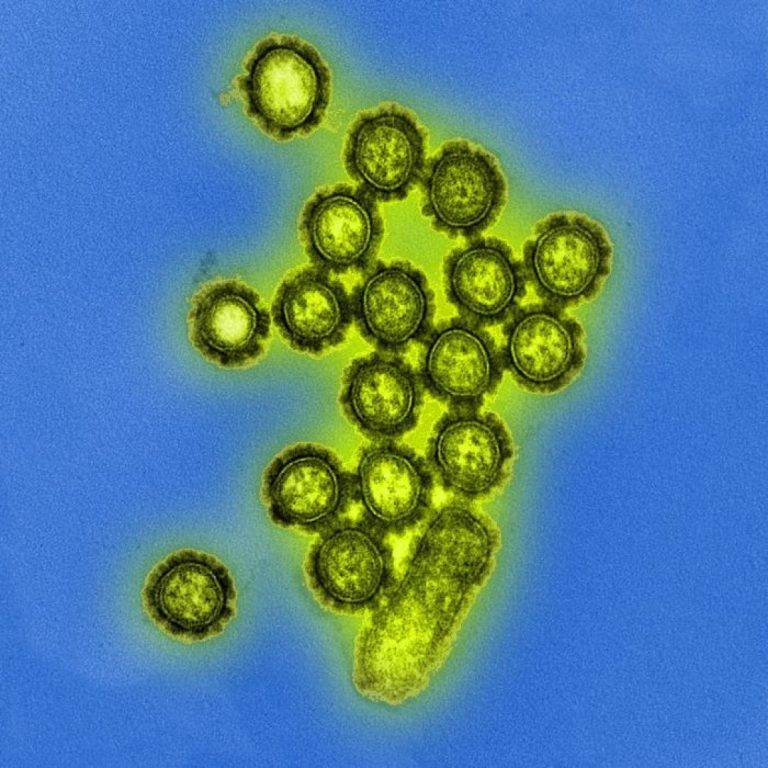
On Dec. 1, 2023, the World Health Organization (WHO) announced that the International Health Regulations National Focal Point…

On Nov. 29, 2023, the World Health Organization (WHO) announced that between 24 and 25 November 2023, the…

On Nov. 28, 2023, Novavax announced that Nuvaxovid XBB.1.5 COVID-19 Vaccine (NVX-CoV2601) had been granted Emergency Use Listing…

On Nov. 23, 2023, the World Health Organization (WHO) announced that it was monitoring data from Chinese surveillance…

On Nov. 21, 2023, the World Health Organization (WHO) reported that the BA.2.86 variant had been reported in…

On Nov. 16. 2023, the U.S. Centers for Disease Control and Prevention (CDC) announced that Following years of…