The Global Polio Laboratory Network was established
In 1990, the Global Polio Laboratory Network (GPLN) was formally established by the World Health Organization (WHO), national…

In 1990, the Global Polio Laboratory Network (GPLN) was formally established by the World Health Organization (WHO), national…
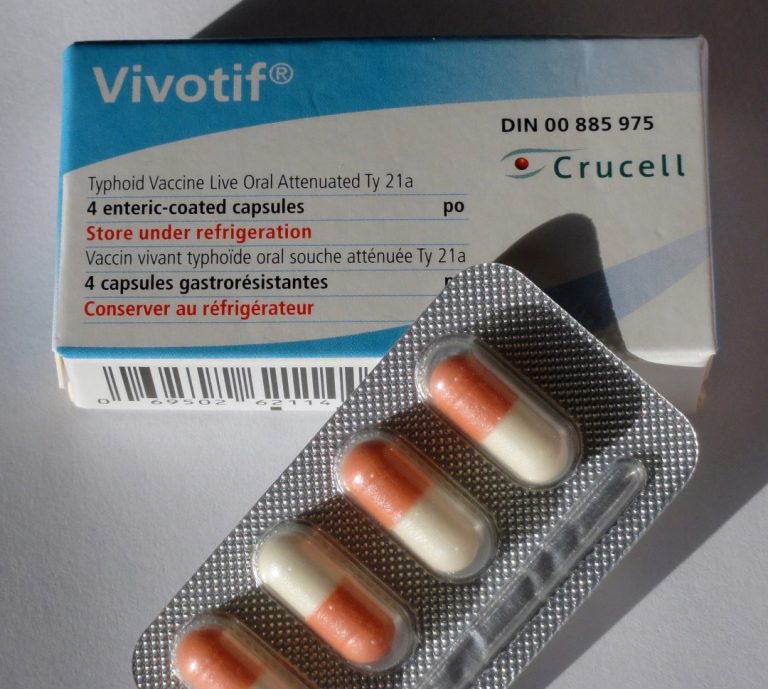
On Dec. 15, 1989, the live, oral typhoid vaccine (Ty21a, Vivotif Berna by Swiss Serum Institute) was licensed….
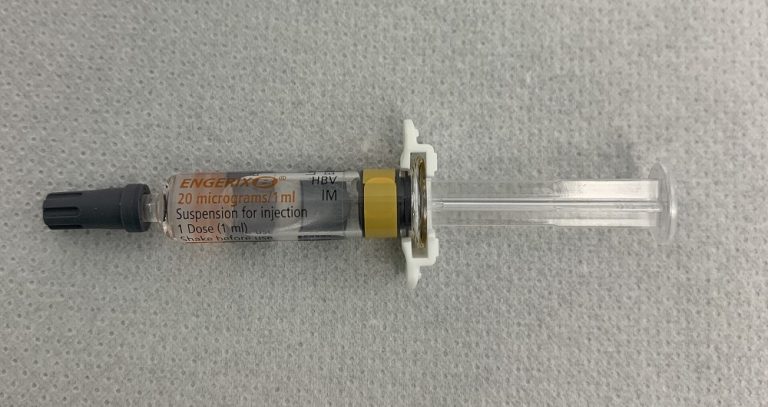
On Aug. 28, 1989, a second recombinant hepatitis B vaccine, Engerix-B (SmithKlineBeecham), was approved for use in the…
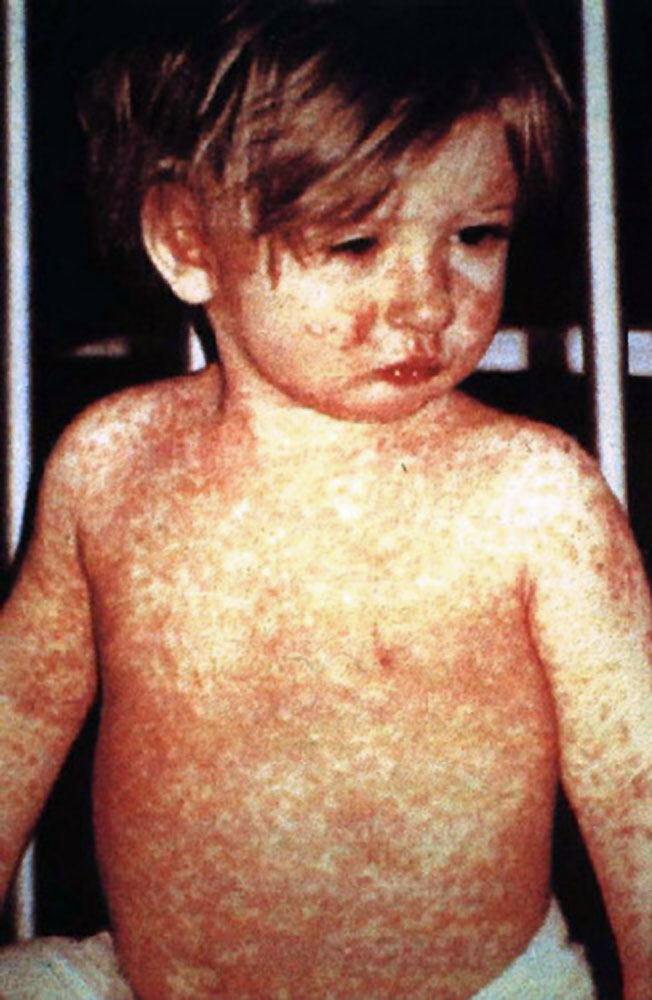
In 1989, recommendations for routine 2nd doses of measles-containing vaccine were issued by both ACIP and the American…

In 1989, the U.S. Centers for Disease Control and Prevention (CDC) established a World Health Organization (WHO) collaborating…

In 1989, the State Office of Rural Health became part of Oregon Health & Science University (OHSU) to…

On Dec. 21, 1988, the conjugated Haemophilus influenzae type b vaccine (HibTITER by Wyeth-Lederle) was licensed. Prior to…
On Jan. 22, 1988, the Centers for Disease Control and Prevention’s (CDC) Immunization Practices Advisory Committee (ACIP) recommended…

In 1988, the World Health Assembly adopted a resolution for the worldwide eradication of polio, marking the launch…
On Dec. 22, 1987, the protein-conjugated Haemophilus influenzae type b vaccine (PRP-D, ProHibit by Connaught) was licensed.

On Nov. 14, 1986, the U.S. Congress created the National Vaccine Program (NVP) to coordinate the vaccine research…
On Jul. 23, 1986, the Recombinant hepatitis B vaccine (Recombivax HB by Merck) was licensed. Using recombinant DNA…

In 1986, while teaching a graduate course at the University of Alberta, Dr. Tyrrell found clues that might…

In 1986, the National Childhood Vaccine Injury Act was enacted by Congress. The Department of Health and Human…

In 1986, The National Childhood Vaccine Injury Act (PDF – 312 KB), as amended, created the National Vaccine Injury…
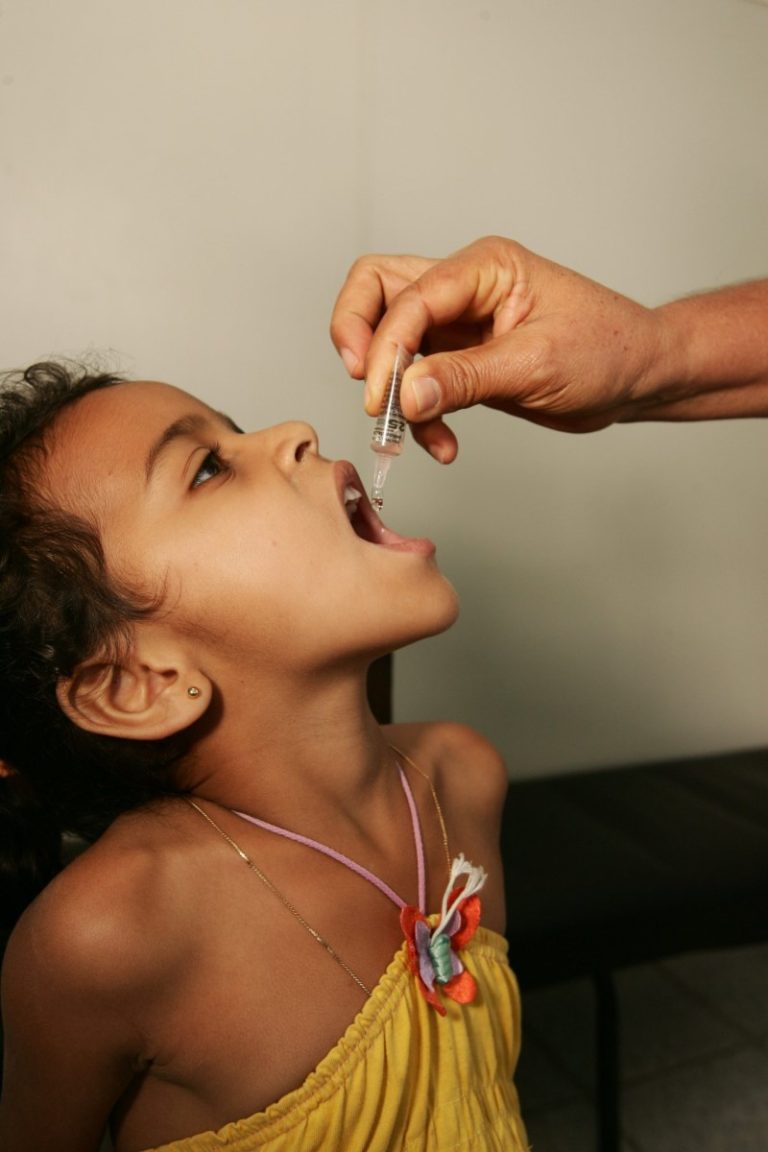
In 1985, Rotary International established its PolioPlus program, which held two fundraising events. Rotary has contributed over $500…

In 1985, the CDIPD was founded as an NIH-NIAID-supported Tropical Disease Research Unit (TDRU) at University of California,…

On Sept. 1, 1984, with the enactment of P.L. 98-369 by the U.S. Congress, coverage under Part B…
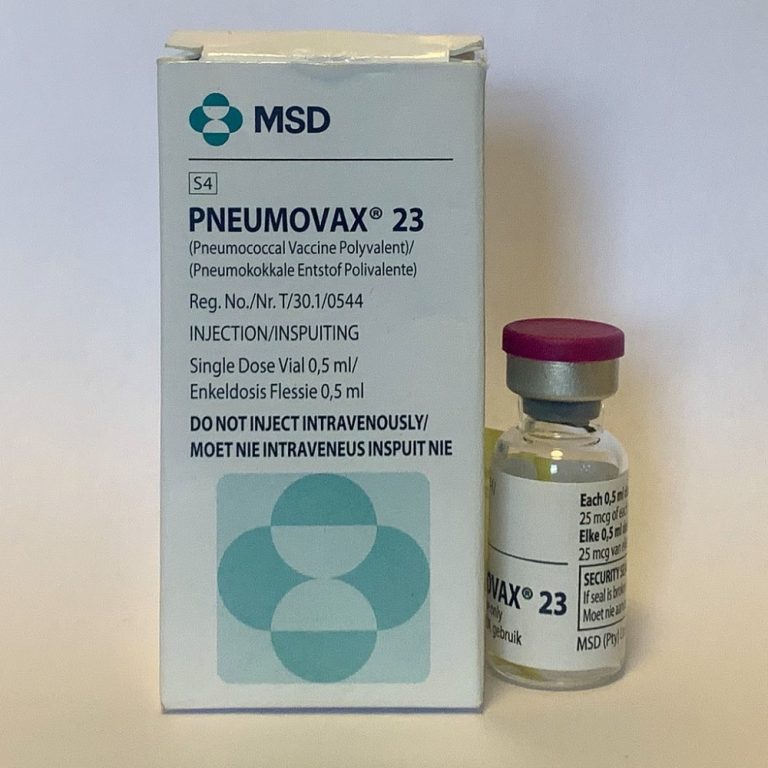
On Jun. 30, 1983, the U.S. Food and Drug Administration (FDA) licensed two enhanced pneumococcal polysaccharide vaccines (Pneumovax…

in May 1983, President Ronald Reagan signed into law a bill that established the Foundation for the Advancement…

In 1983, Hollister-Stier Laboratories became a division of the Miles Pharmaceutical Group. Hollister-Stier Laboratories, located in Spokane, was…

In 1982, the Small Business Innovation Research (SBIR) program, administered by the U.S. Small Business Administration (SBA), was…

In 1982, the first recombinant DNA vaccine for livestock was developed. An advantage of the vaccines made through…

In 1982, the first hepatitis B viral vaccines, developed by Merck and also by the Pasteur Institute, were…

In 1982, the U.S. Centers for Disease Control and Prevention (CDC) reported an all-time low of measles cases,…
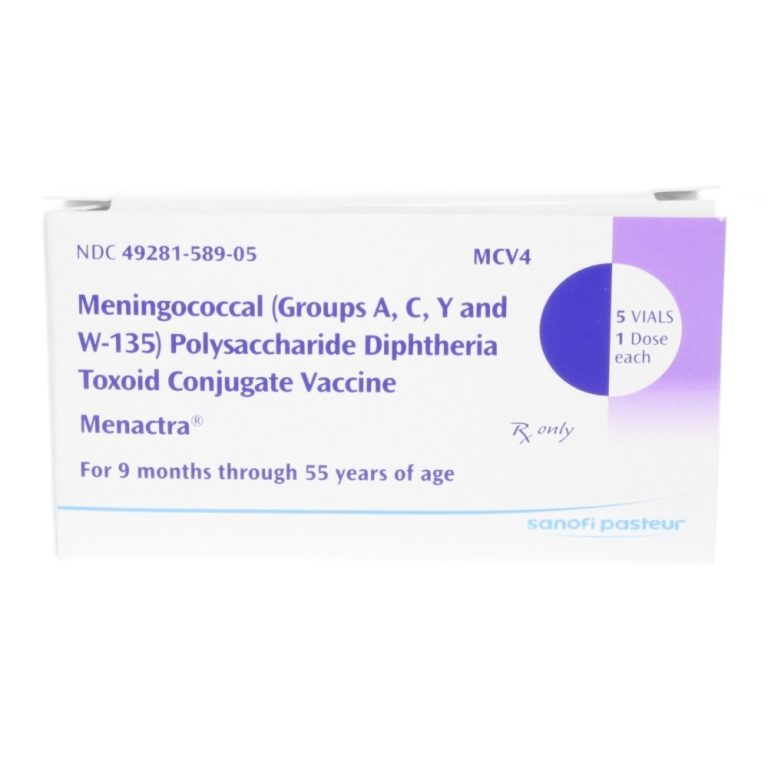
On Nov. 23, 1981, the U.S. Food and Drug Administration licensed Quadrivalent groups A, C, Y, and W-135…

On Jul. 1, 1981, as a result of the enactment of Public Law 96-611 passed by the U.S….

In 1981, the U.S. Food and Drug Administration (FDA) approved a plasma-derived hepatitis B vaccine for human use….

In 1981, the U.S. Centers for Disease Control and Prevention (CDC) began collecting reports of influenza outbreaks from…

On Dec. 12, 1980, the U.S. Congress enacted the Bayh-Dole Act (P.L. 96-517, Patent and Trademark Act Amendments of…