The COVID-19 Vaccine
Cast of Characters: Donald Trump, President | Mike Pence, Vice President | Anthony S. Fauci, MD, Director, National…
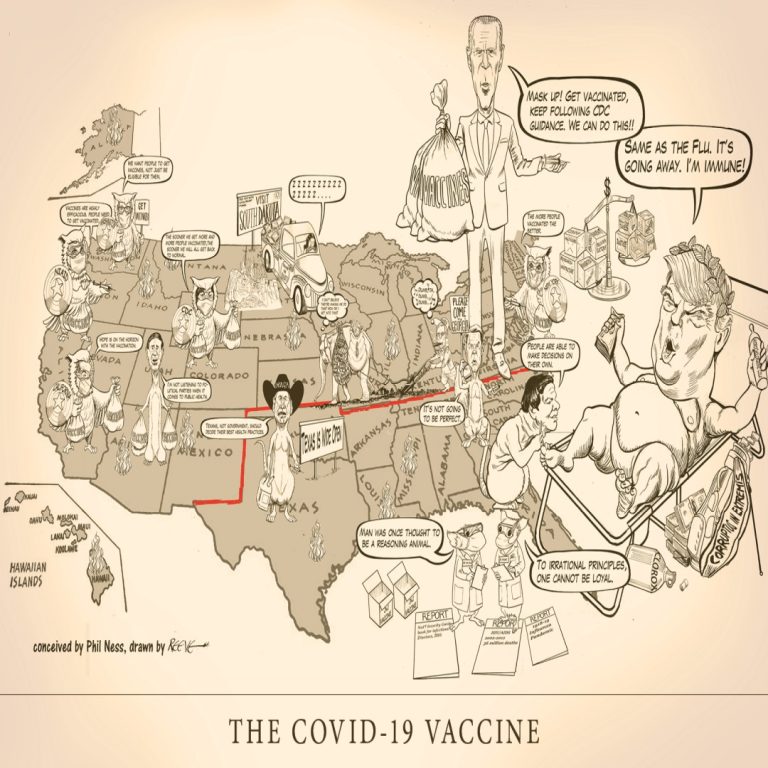
Cast of Characters: Donald Trump, President | Mike Pence, Vice President | Anthony S. Fauci, MD, Director, National…

On Jul. 26, 2021, COVAX and the World Bank accelerated COVID-19 vaccine supply for developing countries through a…

On Jul. 26, 2021, the Department of Veterans Affairs (VA) announced that it had mandated COVID-19 vaccines for…

On Jul. 26, 2021, researchers at the University of Oxford launched a Phase 1 trial to test a…
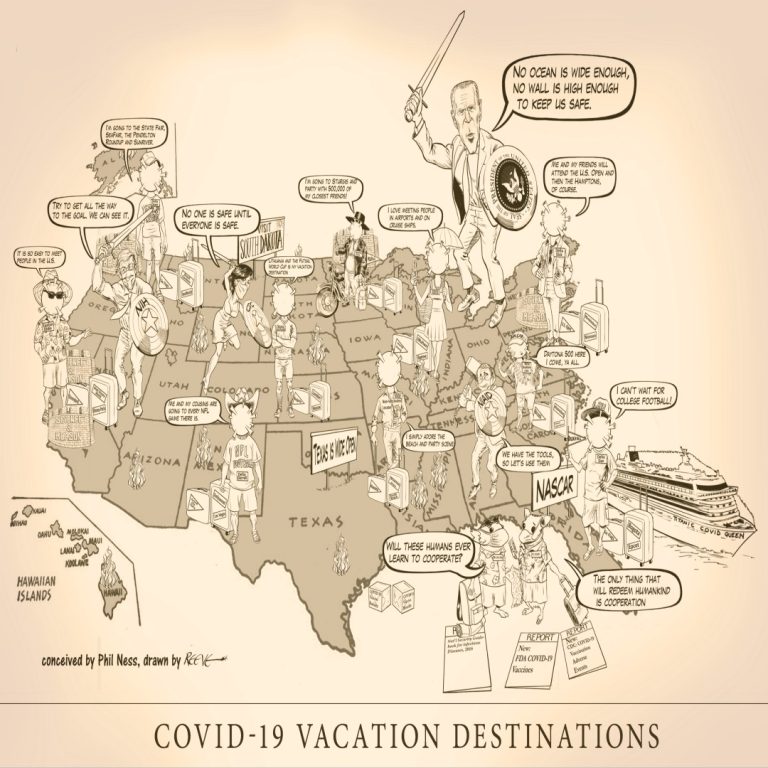
Planning a Summer vacation? Where to go? How about going to one of those large events with tens…

On Jul. 23, 2021, Moderna announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human…
On Jul. 22, 2021, Moderna announced a new supply agreement with Taiwan for 20 million doses of Moderna’s…

On Jul. 21, 2021, Pfizer and BioNTech announced the signing of a letter of intent with The Biovac…

On Jul. 20, 2021, Moderna announced that the Ministry of Health, Labour and Welfare of Japan (MHLW) and…

On Jul. 15, 2021, Corvus Pharmaceuticals announced that it had discontinued its Phase 3 study of mupadolimab for…

On Jul. 15, 2021, iBio announced that preclinical studies of IBIO-202, its subunit vaccine candidate that targets the…

On Jul. 12, 2021, Gavi, the Vaccine Alliance, announced that it had signed advance purchase agreements with Sinopharm…

On Jul. 12, 2021, Moderna announced a supply agreement with the government of Argentina for 20 million doses…

On Jul. 12, 2021, BioNTech announced that Fosun Industrial Co. had reached advance procurement agreements for the mRNA-based…

On Jul. 8, 2021, Pfizer and BioNTech announced encouraging data in the ongoing booster trial of a third…

On Jul. 7, 2021, Rice University announced a study to develop an inhalable COVID-19 vaccine. The project led…

On Jul. 6, 2021, Dynavax Technologies and Biological E announced the execution of a commercial supply agreement of…

On Jul. 1, 2021, Moderna confirmed that following the European Medicines Agency’s (EMA) Committee for Human Medicines (CHMP)…

On Jul. 1, 2021, HDT Bio announced the U.S. Food and Drug Administration had reviewed the companyメs Investigational…

On Jun. 30, 2021, Gavi, the Vaccine Alliance, that it had signed an advance purchase agreement with Clover…
On Jun. 30, 2021, CureVac announced results from the final analysis of its 40,000 subject international pivotal Phase…
On Jun. 30, 2021, the National Institutes of Health announced that two U.S. Phase 1 clinical trials of…

On Jun. 30, 2021, Novavax announced the publication of results from the final analysis of a pivotal Phase…

On Jun. 29, 2021, the National Institutes of Health announced that an adjuvant developed with its funding had…

On Jun. 29, 2021, Paratek Pharmaceuticals announced it had delivered the first procurement of NUZYRA to the Biomedical…

On Jun. 29, 2021, Moderna announced new results from in vitro neutralization studies of sera from individuals vaccinated…

On Jun. 29, 2021, Moderna announced that the government of India had issued a registration certificate and a…

On Jun. 29, 2021, Altimmune announced phase 1 AdCOVID clinical trial data that showed AdCOVID appeared to be…

On Jun. 29, 2021, VBI Vaccines announced positive Phase 1 data from its Phase 1/2 trial of the…

On Jun. 28, 2021, the University of Oxford in partnership with AstraZeneca began vaccinations for a new phase…