CDC issued alert for increased risk of Dengue Virus infections in the U.S.
On Jun. 25, 2024 the Centers for Disease Control and Prevention (CDC) issued a Health Alert Network (HAN)…

On Jun. 25, 2024 the Centers for Disease Control and Prevention (CDC) issued a Health Alert Network (HAN)…

On Jun. 20, 2024, the U.S. Food and Drug Administration expanded the approval of Sarepta Therapeutics’ Elevidys (delandistrogene…

On Jun. 19, 2024, the Institute for Health Metrics and Evaluation (IHME) released a comprehensive new report that…

On Jun. 17, 2024, Merck announced that the U.S. Food and Drug Administration (FDA) had approved CAPVAXIVE™ (Pneumococcal…
On Jun. 14, 2024, the FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) unanimously voted to recommend…

On Jun. 13, 2024, BioMADE announced an investment in a state-of-the-art bioindustrial manufacturing infrastructure in Minnesota, following support…

On Jun. 11, 2024, the National Academies of Sciences, Engineering, and Medicine released a new report that stated the…

On May 31, 2024, a national research coalition led by the University of British Columbia (UBC) announced a…

On May 31, 2024, Moderna announced that the U.S. Food and Drug Administration (FDA) had approved mRESVIA (mRNA-1345),…

On May 30, 2024, the World Health Organization (WHO) reported that as of 30 April 2024, over 7.6…

On May 30, 2024, the California Institute for Regenerative Medicine (CIRM) announced it had awarded $53 million to…

On May 30, 2024, Biogen announced that the European Commission (EC) had granted marketing authorization under exceptional circumstances…

On May 29, 2024, 23andMe with support from The Michael J. Fox Foundation for Parkinson’s Research, announced it…

On May 23, 2024, the U.S. Centers for Disease Control and Prevention (CDC) reported that 109 people from…

On May 15, 2024, the Cancer Prevention and Research Institute of Texas (CPRIT) announced that it had approved…

On May 15, 2024, the American Association for Cancer Research (AACR) released its biennial Cancer Disparities Progress Report…

Asian American and Pacific Islander History Month celebrates the scientific accomplishments of several firsts, from the first chemo…

Montana Life Science Genealogy illustrates life science companies and non-profit research organization in the State of Montana by…
On May 1, 2024, researchers at the National Institutes of Health (NIH) announced a review in JAMA that…

On Apr. 30, 2024, the U.S. Preventive Services Task Force (USPSTF) recommended that all women get screened for…
On Apr. 30, 2024, National Institutes of Health (NIH) researchers and collaborators announced they had discovered over 100…
On Apr. 29, 2024, Oregon Health Sciences University (OHSU) reported a patient with the first documented case of…
On Apr. 26, 2024, the California Institute for Regenerative Medicine (CIRM) announced it had approved awarding nearly $31…
On Apr. 26, 2024, an international team of researchers led by the National Cancer Institute (NCI) released an…
On Apr. 26, 2024, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved BEQVEZ™ (fidanacogene…
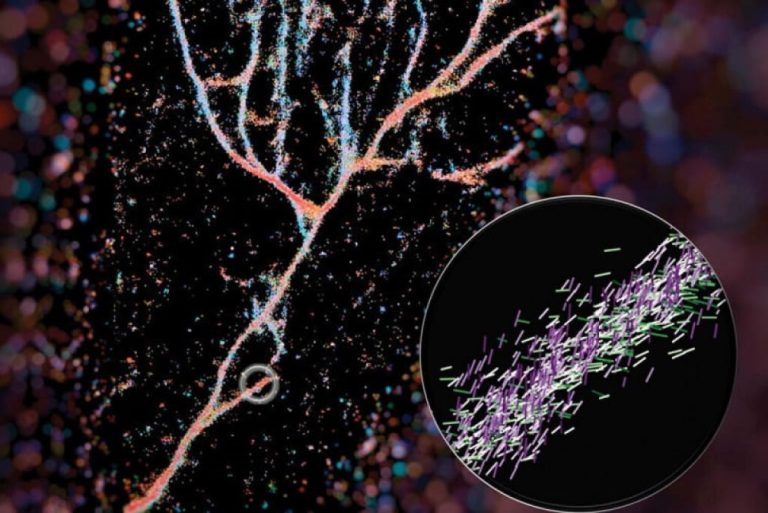
On Apr. 26, 2024, engineers at Washington University in St. Louis announced a new imaging technique that give…
On Apr. 23, 2024, the U.S. Food and Drug Administration (FDA) announced that nearly all (99%) of the…

On Apr. 5, 2024, the U.S. Centers for Disease Control and Prevention (CDC) announced that Texas had reported…

On Apr. 3, 2024, the U.S. Food and Drug Administration approved Basilea Pharmaceutica’s Zevtera (ceftobiprole medocaril sodium for…

On Apr. 1, 2024, Otsuka Pharmaceutica and Click Therapeutics announced that the U.S. Food and Drug Administration (FDA)…