Measles cases surged worldwide, infecting 10.3 million people in 2023
On Nov. 14, 2024, the World Health Organization (WHO) and the U.S. Centers for Disease Control and Prevention…

On Nov. 14, 2024, the World Health Organization (WHO) and the U.S. Centers for Disease Control and Prevention…

On Nov. 14, 2024, the U.S. Food and Drug Administration approved PTC Therapeutics’ Kebilidi (eladocagene exuparvovec-tneq), an adeno-associated…
On Nov. 14, 2024, an analysis from the Global Burden of Disease Study Collaborator Network warned of an…

On Nov. 13, 2024, the NCD Risk Factor Collaboration (NCD-RisC) with support from the World Health Organization (WHO)…

On Nov. 12, 2024, researchers from the University of Basel and University Hospital Basel announced they had developed…

On Nov. 12, 2024, researchers from the University of California, San Francisco (UCSF) reported that their genomic test…
On Nov. 12, 2024, a research team led by UCLA Health Jonsson Comprehensive Cancer Center investigators has shown that…
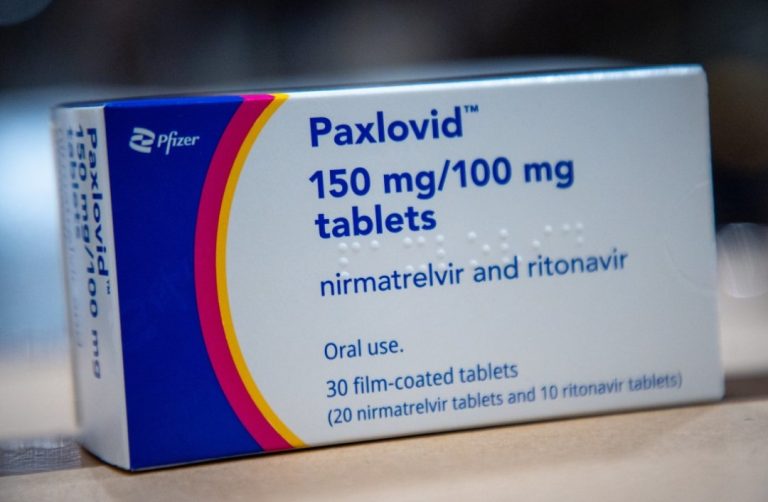
On Nov. 11, 2024, findings from two studies have tied use of the antiviral drug nirmatrelvir-ritonavir (Paxlovid) to…

On Nov. 11, 2024, a team of researchers led by Iowa State University (ISU) have shown that as…
On Nov. 11, 2024, an international team of researchers has for the first time cured patients with Toxic…
On Nov 8, 2024, researchers from the Wellcome Sanger Institute celebrated a milestone of sequencing 50 Petabases of…

On Nov. 8, 2024, as companies begin considering moving their manufacturing operations outside of China in order to…

On Nov. 6, 2024, researchers from the Pasteur Institute in Cambodia published a detailed genetic analysis of the…
On Nov. 6, 2024, researchers from Emory University, in collaboration with scientists at Gladstone Institutes and University of…

On Nov. 5, 2024, Scripps research scientists reported they have shown that Alzheimer’s and alcohol use disorder (AUD)…
On Nov. 5, 2024, a cross-sectional study of data for 36 cancer types from 185 countries and territories…
On Nov. 4, 2024, a team of University of Wisconsin–Madison researchers warned that artificial intelligence tools gaining popularity…
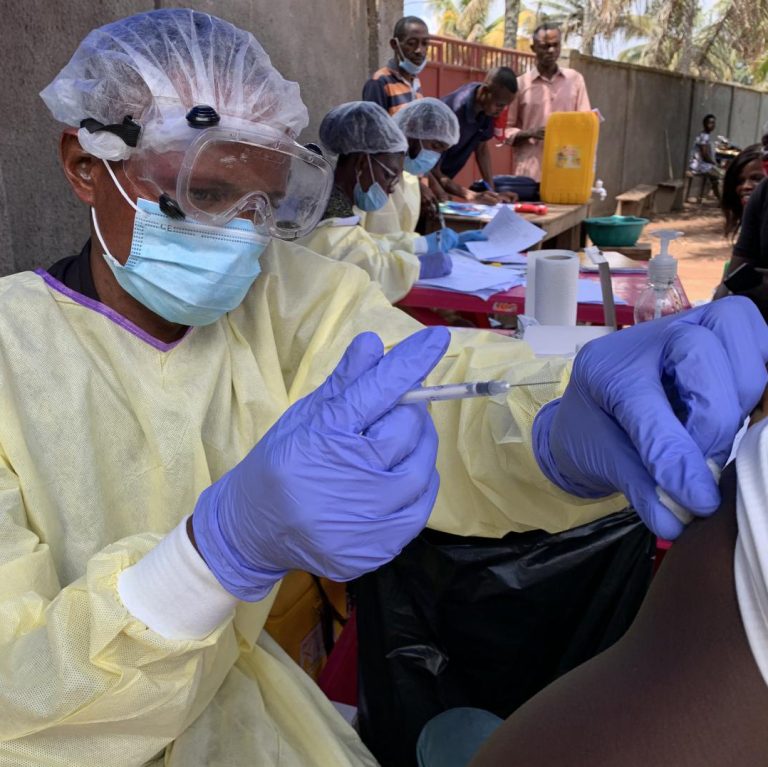
On Nov. 1, 2024, the World Health Organization reported that as of October 31, 66 confirmed cases, including…

On Oct. 31, 2024, researchers from the University of Pittsburgh reported results of a study that showed drug-related…
On Oct. 30, 2024, ten studies from the Human Tumor Atlas Network (HTAN), a National Institutes of Health…
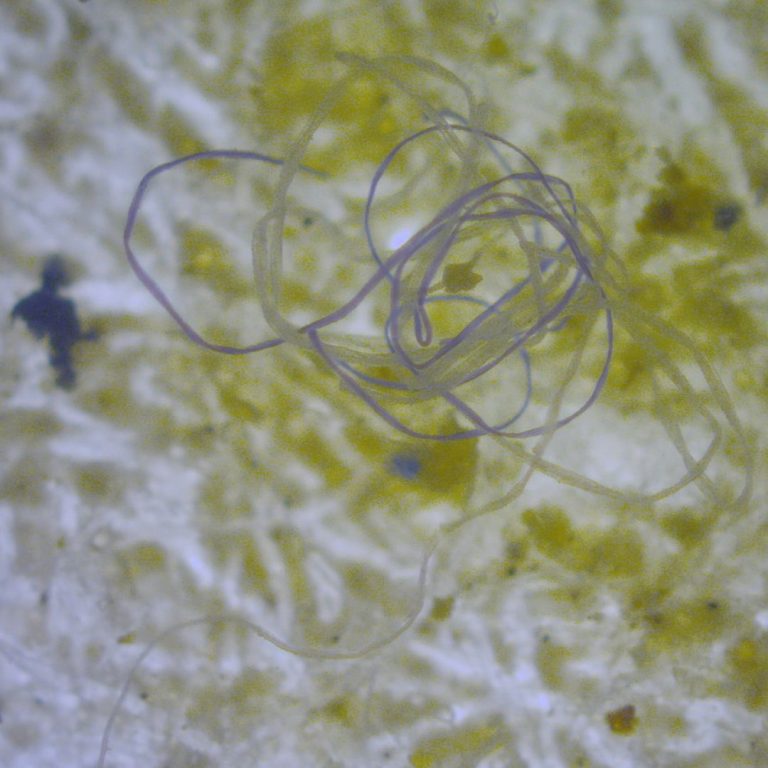
On Oct. 30, 2024, an international research team led by the University of Bonn reported results of a…

On Oct. 29, 2024, a study led by Andrei Gudkov, PhD, DSci, at Roswell Park Comprehensive Cancer Center support…
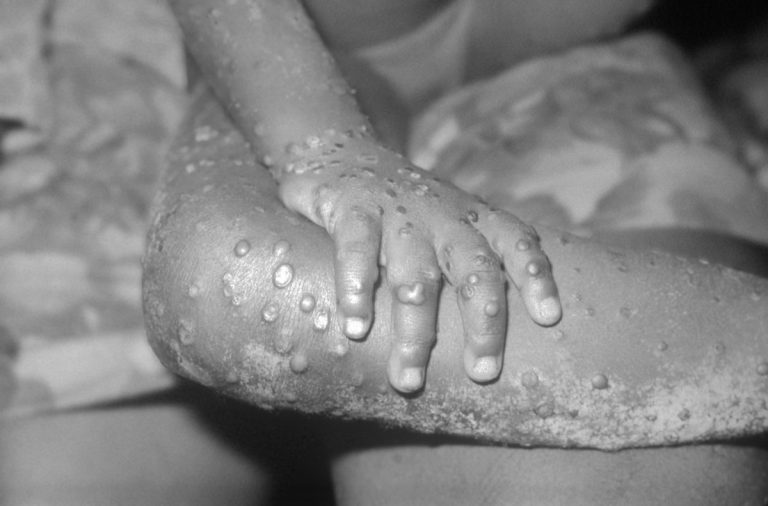
On Oct. 29, 2024, the World Health Organization (WHO) announced that it had, in collaboration with Member States,…

On Oct. 28, 2024, the Johns Hopkins University and Johns Hopkins Medicine, together with descendants of Henrietta Lacks,…

On Oct. 28, 2024, HOPO Therapeutics announced that it has been awarded a contract valued at up to…
On Oct. 28, 2024, the Iowa Department of Health and Human Services (HHS) confirmed the death of a…
On Oct. 28, 2024, University of Wisconsin–Madison researchers reported that a strain of H5N1 avian influenza virus found…

On Oct. 25, 2024, the World Health Organization reported that as of October 24, a total of 64…

On Oct. 24, 2024, researchers from Children’s Medical Center Research Institute at UT Southwestern reported that ancient viral remnants…

On Oct. 23, 2024, the University of Rochester announced that the Saunders Foundation, led by University Trustee Emeritus…