Norovirus cases are surging in parts of the U.S.
On Dec. 28, 2024, the U.S. Centers for Disease Control and Prevention (CDC) reported that cases of a…
On Dec. 28, 2024, the U.S. Centers for Disease Control and Prevention (CDC) reported that cases of a…
On Dec. 24, 2024, the World Health Organization (WHO) announced that SARS-CoV-2, the virus that causes COVID-19, largely…
On Dec. 23, 2024, researchers with the Advanced Science Research Center at the CUNY Graduate Center (CUNY ASRC)…
On Dec. 23, 2024, a study from Radboud University reported that the standard medication levodopa does not always…

On Dec. 23, 2024, an international team of researchers reported results of a study that showed the escalating…
On Dec. 20, 2024, the World Health Organization (WHO) announced that the outbreak of Marburg Virus Disease was…

On Dec. 20, 2024, the U.S. Food and Drug Administration (FDA) approved Eli Lilly’s Zepbound (tirzepatide) for the…

On Dec. 20, 2024, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved BRAFTOVI® (encorafenib)…

On Dec. 20, 2024, Novo Nordisk announced that the U.S. Food and Drug Administration (FDA) had approved Alhemo®…

On Dec. 19, 2024, researchers from The Hospital for Sick Children (SickKids) in Canada and the Istituto Giannina…
On Dec. 19, 2024, a University of Queensland (UQ) study has found no link between exposure to water…
On Dec. 18, 2024, the Institute for Health Metrics and Evaluation reported that diarrhoeal diseases claim more than…

On Dec. 18, 2024, the Barbara Ann Karmanos Cancer Institute announced that the new TheraBionic P1 device, an…

On Dec. 18, 2024, the National Academies of Sciences released a report showing the current information ecosystem makes…

On Dec. 18, 2024, A huge collaboration has confirmed growing concerns that fake or flawed research is polluting…
On Dec. 18, 2024, Caltech researchers announced they had developed a new method to map the positions of…

On Dec. 18, 2024, BiVACOR, a clinical-stage medical device company, announced the successful completion of the first phase…

On Dec. 18, 2024, Gavin Newsom, Governor of Californa, proclaimed a State of Emergency to streamline and expedite…

On Dec. 18, 2024, a study that stoked enthusiasm for the now-disproven idea that the cheap malaria drug…

On Dec. 18, 2024, the U.S. Centers for Disease Control and Prevention (CDC) reported a study with patient…

On Dec. 17, 2024, scientists from Hiroshima University announced they had mapped near-gap-free and near-error-free genomes of a…

On Dec. 17, 2024, United Therapeutics, a public benefit corporation, announced the world’s first transplant of a UKidney,…
On Dec. 17, 2024, scientists at The University of Queensland (UQ) announced they had licensed a promising ultrasound…
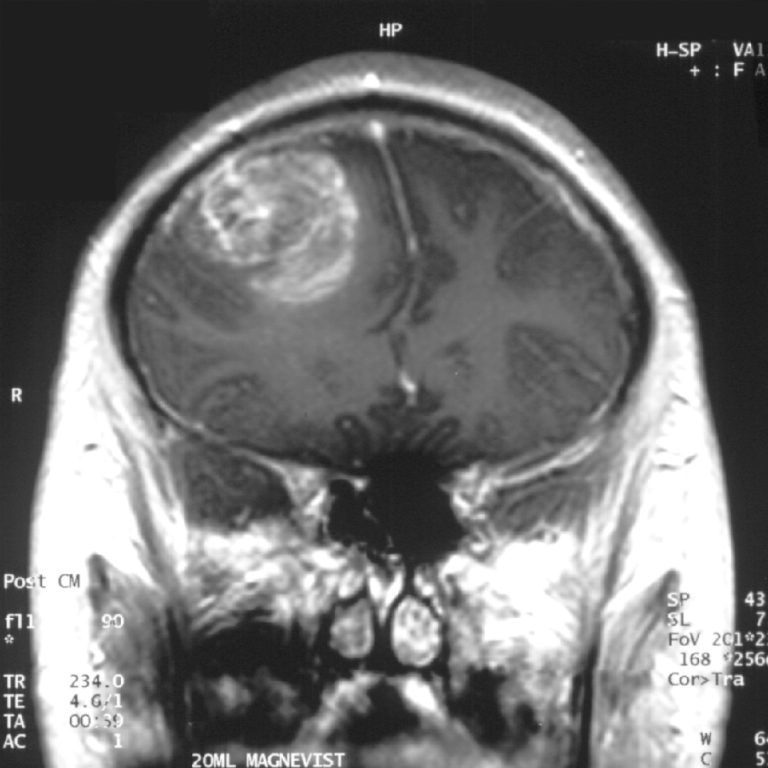
On Dec. 16, 2024, the Mayo Clinic announced the results of an innovative treatment approach that may offer…

On Dec. 16, 2024, a study published in BMC Cardiovascular Disorders shows that pediatric and young adult COVID-19…

On Dec. 11, 2024, Massachusetts General Hospital (MGH), founding member of the Mass General Brigham healthcare system, announced…

On Dec. 11, 2024, University of Pennsylvania Engineers announced they had made a critical breakthrough that bridges a…

On Dec. 9, 2024, neurologists at Fudan University in Shanghai, China have identified 13 proteins in the blood…

On Dec. 9, 2024, the Africa Centers for Disease Control (CDC) announced that it is working closely with…

On Dec. 5, 2024, the California Department of Public Health (CDPH) reported that following an investigation with the…