U.S. healthcare spending soars to over $5 trillion in 2024
On Jan. 14, 2026, U.S. healthcare spending rose by 7.2% to $5.3 trillion in 2024 from $4.9 trillion…

On Jan. 14, 2026, U.S. healthcare spending rose by 7.2% to $5.3 trillion in 2024 from $4.9 trillion…
On Jan. 8, 2026, researchers reported results from a study on the largest cohort of autobrewery syndrome (ABS)…

On Dec. 19, 2025, BioMarin Pharmaceutical and Amicus Therapeutics announced that BioMarin has entered into a definitive agreement…

On Dec. 11, 2025, the California Institute for Regenerative Medicine (CIRM) governing board approved funding of over $160…

On Nov. 24, 2025, a new AI model called popEVE developed by Harvard Medical School researchers and colleagues…

On Nov. 20, 2025, the National Institutes of Health (NIH) has canceled funding for at least 383 clinical…

On Nov. 18, 2025, Arrowhead Pharmaceuticals announced that the U.S. Food and Drug Administration (FDA) has approved REDEMPLO…

On Nov. 17, 2025, a JAMA published study found that more than 74,000 people have had their lives…

On Oct. 15, 2025, Danish drugmaker Novo Nordisk and Omeros announced they have signed a licensing deal worth…

On Jul. 9, 2025, as part of a new study, neurologists at NYU Langone Health announced they have…

On May 20, 2025, the World Health Organization (WHO) announced that world leaders pledged at least an additional…

On May 19, 2025, Prime Medicine announced positive initial data from the first patient dosed in its ongoing…

On May 15, 2025, a team at Children’s Hospital of Philadelphia (CHOP) and Penn Medicine announced that a…

On Apr. 1, 2025, layoff notices began arriving for thousands of employees of the sprawling U.S. Department of…
On Mar. 28, 2025, the U.S. Food and Drug Administration (FDA) announced approval of Alnylam Pharmaceuticals Qfitlia™ (fitusiran),…

On Mar. 27, 2025, a Nature survey of more than 1,200 scientists, three-quarters of the total respondents are…

On Mar. 20, 2025, Alnylam Pharmaceuticals announced the U.S. Food and Drug Administration (FDA) approval of the supplemental…
On Mar. 6, 2025, the U.S. Food and Drug Administration (FDA) announced the approval of ENCELTO (revakinagenetaroretcel-lwey), a…
On Feb. 19, 2025, scientists at St. Jude Children’s Research Hospital report results from a promising new approach…

On Jan. 14, 2025, the U.S. Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) announced…
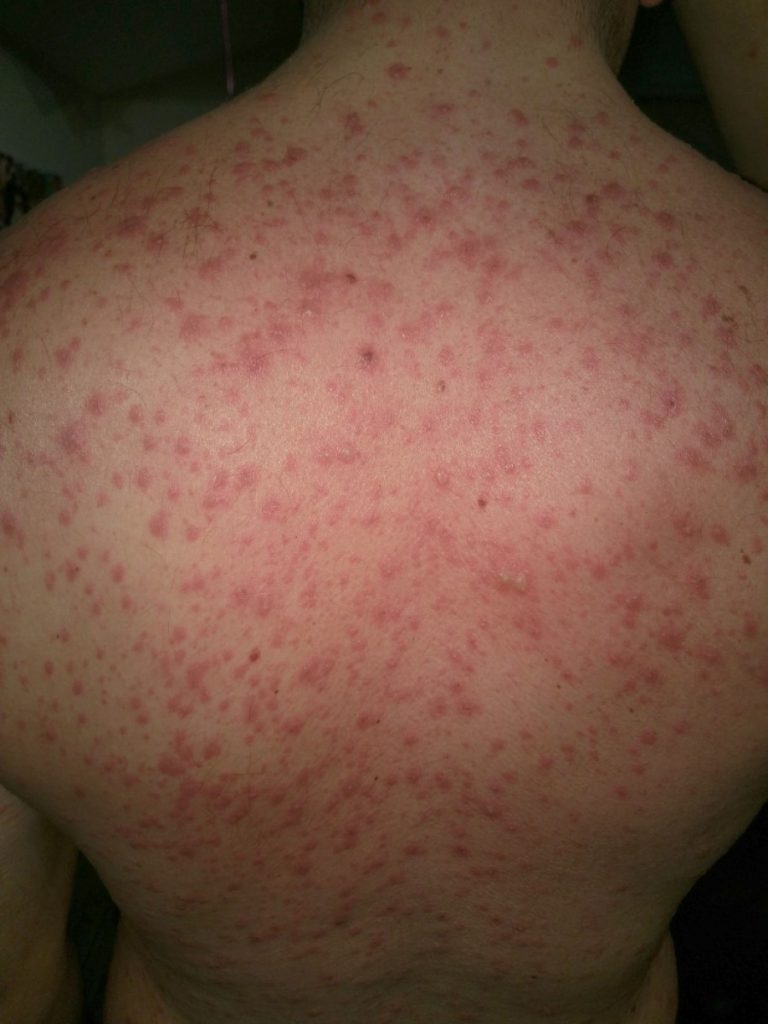
On Nov. 11, 2024, an international team of researchers has for the first time cured patients with Toxic…

On Oct. 3, 2024, the National Health Service England (NHS) announced that Hundreds of babies have begun to…
On Oct. 3, 2024, scientists announced the largest whole-exome sequencing study of epilepsy to date, with more than…

On Sept. 25, 2024, a study led by scientists at Harvard Medical School announced they had developed an…
On Sept. 19, 2024, the World Health Organization (WHO) announced that Jordan had become the first country in…

On Jun. 29, 2023, the U.S. Food and Drug Administration approved Roctavian, an adeno-associated virus vector-based gene therapy…

On Apr. 26, 2021, Moderna announced that it had entered into an agreement with Sanofi for fill/finish sterile…

On Nov. 23, 2020, the U.S. Food and Drug Administration (FDA) approved Alnylam Pharmaceuticals’s Oxlumo (lumasiran) as the…

On Sept. 30, 2020, the National Institutes of Health (NIH) announced funding of a nationwide study to discover…

On Apr. 13, 2020, the U.S. Food and Drug Administration (FDA) announced that given the increased demand for…