California’s Stem Cell Agency invested $25 million for discovery and preclinical research, patient support program
On Mar. 29, 2024, the California Institute for Regenerative Medicine (CIRM) announced awards that support 11 projects in…

On Mar. 29, 2024, the California Institute for Regenerative Medicine (CIRM) announced awards that support 11 projects in…

On Feb. 23, 2024, the California Institute for Regenerative Medicine (CIRM), the world’s largest institution dedicated to regenerative…
On Feb. 16, 2024, the U.S. Centers for Disease Control and Prevention announced that ten people infected with…

On Feb. 9, 2024, the state of Alaska Department of Health Alaska Department of Health confirmed the first…

On Jan. 5, 2024, the U.S. Food and Drug Administration (FDA) authorized Florida’s Agency for Health Care Administration’s…

On Dec. 14, 2023, Biogen and Sage Therapeutics announced ZURZUVAE (zuranolone) 50 mg (two 25 mg capsules per…

On Nov. 22, 2023, the U.S. Food and Drug Administration (FDA) issued the final guidance COVID-19: Developing Drugs…

On Nov. 13, 2023, the White House announced the first-ever Initiative on Womenメs Health Research, an effort led…

On Sept. 29, 2023, the California Institute for Regenerative Medicine (CIRM) awarded $43.8 million to fund projects aimed…

On Sept. 12, 2023, the National Institutes of Health announced it was establishing the Multi-Omics for Health and…
On Aug. 24, 2023, NIAID reported that emerging resistance to common malaria treatments in Uganda could be connected…
On Aug. 11, 2023, a study funded by the National Institute of Allergy and Infectious Diseases (NIAID) reported…

On Aug. 7, 2023, the United Kingdom Health and Security Agency announced the Vaccine Development and Evaluation Centre…

On Aug. 7, 2023, a National Institute of Health supported study found that in 2021, an estimated 2.5…

On Jun. 22, 2023, the U.S. Food and Drug Administration (FDA) approved Sarepta Therapeutics’s Elevidys, the first gene…

On May 31, 2023, the California Institute for Regenerative Medicine (CIRM) awarded $10 million to five facilities as…

On Mar. 17, 2023, PrairiesCan announced $80,514,000 over five years to support the Canadian Critical Drug Initiative and…
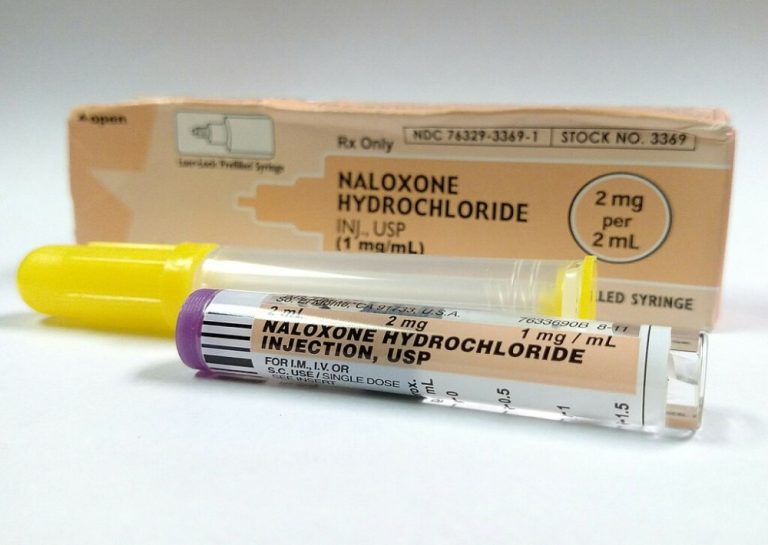
On Mar. 8, 2023, Amphastar Pharmaceuticals announced that the U.S. Food and Drug Administration (FDA) had granted approval…
On Jan. 25, 2023, it was reported that adults living in rural areas of the United States have…

On Nov. 18, 2022, Pfizer and BioNTech announced results from an analysis examining the immune response induced by…

On Oct. 3, 2022, the Nobel Prize in Physiology or Medicine was awarded to was awarded to Svante…

On Aug. 31, 2022, a new front-door to the University of Victoria opened to advance health and life…

On Jul. 28, 2022, AlphaFold, in partnership with EMBL’s European Bioinformatics Institute (EMBL-EBI), announced they we’re releasing predicted…
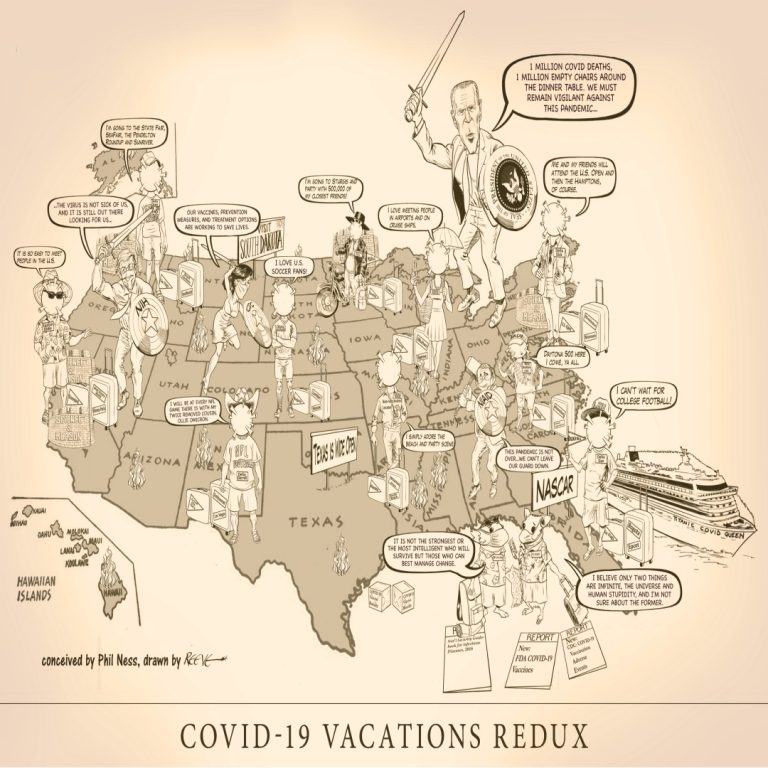
COVID-19 Vacations Redux iIlustrates once again the pits and perils vacation travelers face as they begin their Summer…
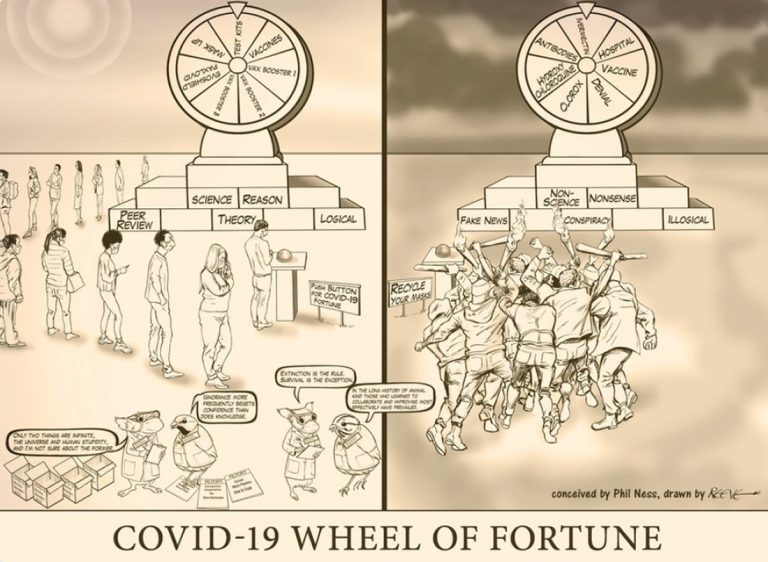
Play the COVID-19 Wheel of Fortune and see how lucky you are! You have two wheels to choose…

On Jun. 2, 2022, the National Institutes of Health (NIH) reported that the Age-Related Eye Disease Studies (AREDS…
On Apr. 1, 2022, Merck and Ridgeback Biotherapeutics announced that data evaluating LAGEVRIO(molnupiravir), an investigational oral antiviral COVID-19…

On Mar. 21, 2022, in support of the Cancer Moonshot goal of fostering data sharing in cancer research,…
On Feb. 2, 2022, President Biden announced a reignition of the Cancer Moonshot, that highlighted new goals: to…

On Jan. 28, 2022, Merck and Ridgeback Biotherapeutics announced data from six preclinical studies demonstrating that molnupiravir, an…