China creates new visa for young STEM talent in national tech drive
On Aug. 14, 2025, China announced it had launched a new visa category for young science and technology…

On Aug. 14, 2025, China announced it had launched a new visa category for young science and technology…

On Aug. 10, 2025, computer scientists at ETH Zurich announced they have developed a digital tool capable of…

On Jul. 29, 2025, Texas A&M study finds immersive VR nature experiences improve mood and quality of life….

On Jul. 28, 2025, a study published in Nature Neuroscience shows that potential contact with approaching infectious avatars,…

On Jul. 25, 2025, Simtra BioPharma announced the purchase from Cook Group of a 65-acre property (301 N….

On Jul. 24, 2025, The China Times reported that China is making inroads in the race for innovative…

On Jul. 18, 2025, researchers from the University of Southern California (USC) reported a wearable sensor could vastly…

On Jul. 14, 2025, Lab equipment maker Waters Corp said it will buy a bioscience and diagnostics unit…

On Jul. 14, 2025, researchers from North Carolina State University (NCSU) have demonstrated a new technique that allows…

On Jul. 10, 2025, MIT researchers announced they have developed a new bionic knee that can help people…
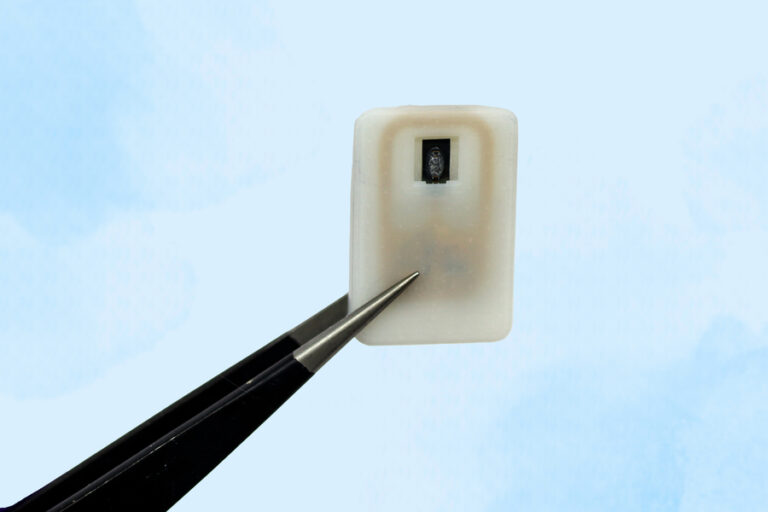
On Jul. 9, 2025, MIT engineers announced they have designed an implantable reservoir that can remain under the…

On Jul. 1, 2025, an international team of researchers led by by the Université de Poitiers and Cardiff…

On Jun. 25, 2025, the National Institutes of Health (NIH) moved to reinstate about 900 grants that a…

On Jun. 23, 2025, Texas A&M research shows wearable health tech can detect infections like COVID-19 and flu…

On Jun. 18, 2025, researchers at the University of Washington (UW) School of Medicine announced that a highly…

On Jun. 13, 2025, the Japanese government unveiled a 100 billion yen ($700 million) policy package designed to…

On Jun. 11, 2025, researchers at the University of California, Davis, announced they have developed an investigational brain-computer…

On Jun. 4, 2025, the Conference of State Parties (CoSP) of the African Medicines Agency (AMA) announced the…

On Jun. 3, 2025, the U.S. Army Combat Capabilities Development Command Chemical Biological Center, DEVCOM CBC, and the…

On Jun. 2, 2025, the U.S. Food and Drug Administration (FDA) announces it has launched Elsa, a generative…

On May 29, 2025, Broad Institute researchers announced they have developed a technology that provides new insight into…
On May 27, 2025, a research team from the University of Washington Medical Center and the University of…

On May 22, 2025, an interdisciplinary group of researchers at UW Medicine and the University of Washington announced…

On My 22, 2025, team led by scientists at the University of Science and Technology of China (USTC)…

On May 16, 2025, the U.S. Food and Drug Administration has cleared Fujirebio Diagnostics’ blood test to diagnose…
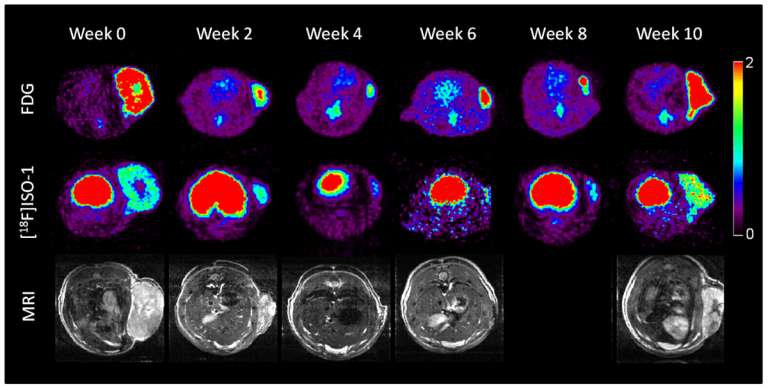
On May 6, 2025, in a first-of-its-kind surgery, a multidisciplinary team led by a University of Maryland Medical…

On May 5, 2025, The European Union (EU) and France announced half a billion euros worth of incentives…
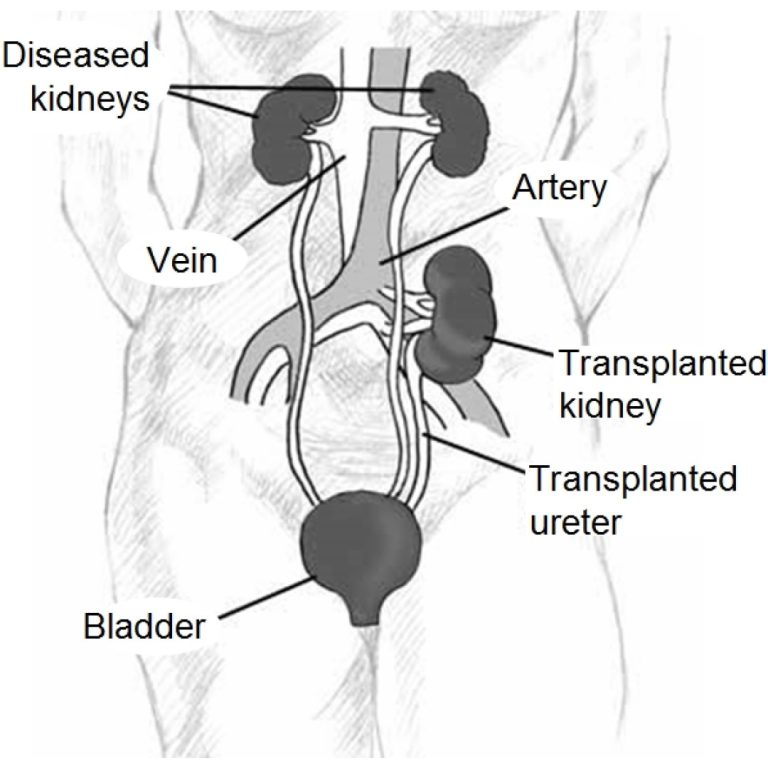
On Apr. 28, 2025, researchers at King’s College London, the University of Bristol and Great Ormond Street Hospital…

On Apr. 9, 2025, Roswell Park Comprehensive Cancer Center achieved textbook outcomes for 90% of 150 consecutive robot-assisted,…

On Apr. 9, 2025, Tulane University researchers announced they have developed a first-of-its-kind handheld diagnostic device that can…