GSK and CureVac announced strategic mRNA technology collaboration
On Jul. 21, 2020, GlaxoSmithKline and CureVac announced a strategic collaboration agreement for the research, development, manufacturing and…

On Jul. 21, 2020, GlaxoSmithKline and CureVac announced a strategic collaboration agreement for the research, development, manufacturing and…

On Jul. 21, 2020, Bio-Techne announced that Exosome Diagnostics, a Bio-Techne brand, had completed validation testing for COVID-19…

On Jul. 20, 2020, BioReference Laboratories, an OPKO Health company, announced accepted an Indefinite Delivery Indefinite Quantity contract…

On Jul. 20, 2020, Pfizer and BioNTech announced an agreement with the United Kingdom to supply 30 million…

On Jul. 20, 2020, BioNTech and Pfizer announced initial data from their ongoing German Phase 1/2, open-label, non-randomized,…

On Jul. 20, 2020, Luminex announced the FDA had issued an Emergency Use Authorization for the company’s xMAPᆴ…

On Jul. 20, 2020, scientists at HDT Bio, PAI Life Sciences, and the University of Washington announced they…

On Jul. 20, 2020, AstraZeneca announced that Interim results from the ongoing Phase I/II COV001 trial, led by…

On Jul. 20, 2020, a scientific analysis of more than 2,000 brain scans found evidence for highly reproducible…

On Jul. 20, 2020, the Serum Institute of India (SII) announced that with the trials of the COVID-19…

On Jul. 20, 2020, interim results from the ongoing Phase I/II COV001 trial, led by Oxford University, showed…

On Jul. 20, 2020, Sorrento announced that it had entered into a binding term sheet for an exclusive…

On Jul. 20, 2020, Innovation Pharma reported receiving new data from ongoing lab testing being conducted at a…

On Jul. 19, 2020, HDT Bio announced co-development efforts with Gennova Biopharmaceuticals to start evaluating HDT-301, a COVID-19…

On Jul. 18, 2020, the FDA reissued an emergency use authorization (EUA) to Quest Diagnostics to authorize its…

On Jul. 18, 2020, a Health Trendsル study from researchers at Quest Diagnostics and the University of Pittsburgh…

On Jul. 16, 2020, Novartis announced a new initiative to help patients in low-income and lower-middle-income countries (LIC;…

On Jul. 16, 2020, Heron Therapeutics announced initiation of the GUARDS-1 Study, a Phase 2 clinical study evaluating…

On Jul. 16, 2020, Catalent and Humanigen announced the expansion of their relationship, under which Catalent will provide…

On Jul. 16, 2020, Codex DNA announced the release of a new synthetic SARS-CoV-2 genome developed to accelerate…

On Jul. 16, 2020, the Tufts Center for the Study of Drug Development reported that although regulatory approval…

On Jul. 16, 2020, Atossa Therapeutics announced that it has contracted with Avance Clinical to conduct a clinical…
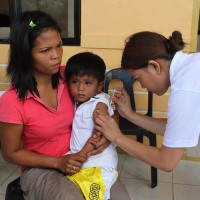
On Jul. 14, 2020, the WHO and UNICEF warned of an alarming decline in the number of children…

On Jul. 15, 2020, in collaboration with the Guangdong CDC, Hunan CDC, Sun Yat-Sen University and Southeast University,…
On Jul. 15, 2020, BioAegis Therapeutics announced that it received regulatory clearance from The Spanish Agency for Medicines…

On Jul. 15, 2020, four Einstein and Montefiore research teams received pilot funding from Einsteinメs Office of the…

On Jul. 14, 2020, in an editorial published in the Journal of the American Medical Association (JAMA), the…

On Jul. 14, 2020, in an editorial published in the Journal of the American Medical Association (JAMA), the…

On Jul. 14, 2020, researchers at the The University of Hong Kong (HKUMed) collaborated with researchers at the…

On Jul. 14, 2020, Shanghai Fosun Pharmaceutical announced it had received the acceptance notice of clinical trial application…