In a first for imaging, new microscope captured details, 3D motion of molecules in liquid
On Feb. 16, 2022, the McKelvey School of Engineering at Washington University in St. Louis, announced that it…

On Feb. 16, 2022, the McKelvey School of Engineering at Washington University in St. Louis, announced that it…

On Feb. 11, 2022, Pfizer and BioNTech announced plans to extend their rolling submission to the U.S. Food…

On Feb. 11, 2022, Roche announced that Actemraᆴ/RoActemraᆴ (tocilizumab) intravenous (IV) had been granted World Health Organization (WHO)…

On Feb. 11, 2022, the WHO announced the addition of tocilizumab, a monoclonal antibody, to its list of…
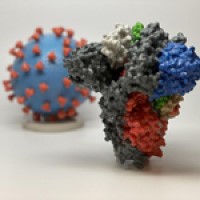
On Feb. 10, 2022, Scripps Research announced that the COVID-causing virus SARS-CoV-2 harbors a vulnerable site at the…

On Feb. 10, 2022, Vir Biotech announced that preclinical data suggested that sotrovimab, an investigational monoclonal antibody authorized…

On Feb. 8, 2022, Howard Hughes Medical Institute (HHMI) announced The program, named Codetta, can read the genome…

On Feb. 7, 2022, scientists working with the Queen Mary University of London and the London Genome Centre…

On Feb. 4, 2022, the NIH announced a study that showed pregnant women with COVID-19 appeared to be…

On Feb. 4, 2022, pet hamsters probably carried the Delta variant of SARS-CoV-2 into Hong Kong and sparked…

On Feb. 4, 2022, the NIH reported that Staphylococcus epidermidis produces enzymes, known as sphingomyelinase, that help the…

On Feb. 4, 2022, a new ᆪ1.6m collaborative project was launched to rapidly identify new treatments for COVID-19….

On Feb. 3, 2022, Novavax announced that the Medicines and Healthcare products Regulatory Agency had granted conditional marketing…

On Feb. 2, 2022, the U.S. Food and Drug Administration announced it had approved the first generic of…
On Feb. 2, 2022, President Biden announced a reignition of the Cancer Moonshot, that highlighted new goals: to…

On Feb. 1, 2022, Pfizer and BioNTech announced that following a request from the U.S. Food and Drug…

On Jan. 31, 2022, Veru announced that the U.S. Food and Drug Administration (FDA) had granted Fast Track…
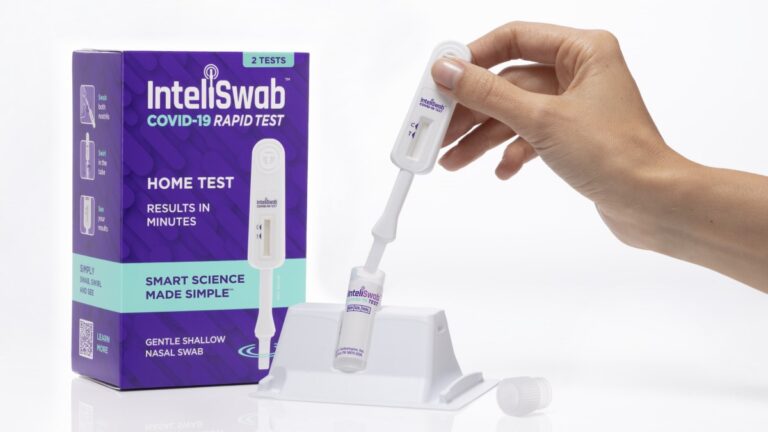
On Jan. 31, 2022, OraSure Technologies announced that its InteliSwab COVID-19 rapid tests had been authorized by the…

On Jan. 28, 2022, the WHO announced that COVID-19 information had reached 1,292,209 people through Viamoメs 3-2-1 Platform…
On Jan. 27, 2022, NIAID announced that a clinical trial found that the combination of remdesivir plus a…

On Jan. 26, 2022, the NIH announced that adults who had previously received a full regimen of any…

On Jan. 25, 2022, Pfizer and BioNTech announced the initiation of a clinical study to evaluate the safety,…

On Jan. 25, 2022, the U.S. Department of Agricultureメs National Veterinary Services Laboratories announced a study with zoos…

On Jan. 25, 2022, researchers at the University of Missouri announced they had identified the highly prevalent, specific…

On Jan. 25. 2022, a report published in PNAS provided strong evidence of extensive SARS-CoV-2 infection of white-tailed…

The Evolution of CRISPR illustrates its climb out of the primordial ocean on the backs of eukaryotic cells…

On Jan. 24, 2022, the University of Oxford announced that a third booster dose of either the ChAdOx1…

On Jan. 20, 2022, Gilead Sciences announced that the U.S. Food and Drug Administration had granted expedited approval…

On Jan. 20, 2022, the National Institutes of Health announced it was awarding $170 million over five years,…

On Jan. 18, 2022, Sorrento Therapeutics announced receipt of clearance from the Brazilian regulatory agency (ANVISA) to proceed…