INOVIO to develop DNA-encoded monoclonal antibody (dMAb) candidates to treat COVID-19
On Dec. 15, 2020, Inovio Pharma announced the company and a team of scientists from The Wistar Institute,…

On Dec. 15, 2020, Inovio Pharma announced the company and a team of scientists from The Wistar Institute,…

On Dec. 15, 2020, Aurinia Pharmaceuticals announced the funding and initiation of an open-label exploratory trial evaluating the…
On Dec. 14, 2020, the U.S. Dept. of Veterans Affairs (VA) announced that the New Orleans and Bedford,…
On Dec. 14, 2020, Anixa Biosciences announced that it and partner OntoChem GmbH had verified that one of…

On Dec. 14, 2020, Moderna confirmed that the Company had concluded an agreement with the Ministry of Health…
On Dev. 11, 2020, the University of Oxford reported that an Artificial Intelligence test had been shown to…

On Dec. 11, 2020, Moderna announced that the U.S. government has exercised its option to purchase an additional…

On Dec. 10, 2020, Roche announced a partnership with Moderna to utilise the Elecsys Anti-SARS-CoV-2 S antibody test…

On Dec. 10, 2020, ImmunityBio announced its COVID-19 vaccine candidate protected nasal and lung airways of non-human primates…

On Dec. 10, 2020, Moderna announced that the first adolescent participants had been dosed in the Phase 2/3…

On Dec. 9, 2020, Roche announced a partnership with Moderna to utilise the Elecsys Anti-SARS-CoV-2 S antibody test…

On Dec. 8, 2020, Moderna announced the Swiss Federal Government had increased its confirmed order commitment from 4.5…

On Dec. 7, 2020, Inovio Pharmaceuticals announced it had dosed its first subject in a Phase 2 clinical…

On Dec. 7, 2020, an universal influenza virus vaccine, which produces antibodies that target the part of the…

On Dec. 7, 2020, Moderna announced that the Canadian Government had increased its confirmed order commitment by 20…

On Dec. 4, 2020, the U.S. Food and Drug Administration (FDA) authorized the first diagnostic test for at home collection…

On Dec. 4, 2020, it was reported in JAMA that high-dose influenza (commonly known as flu) vaccines are…

On Dec. 3, 2020, Moderna announced in a letter to the editor published in the New England Journal…

On Dec. 3, 2020, Inovio Pharmaceuticals announced the execution of an agreement with Kaneka Eurogentec an affiliate of…

On Dec. 2, 2020, the University of Oregon opened the Phil and Penny Knight Campus for Accelerating Scientific…

On Nov. 30, 2020, Moderna announced that the primary efficacy analysis of the Phase 3 study of mRNA-1273…

On Nov. 30, 2020, Novavax announced that two of the three planned late-stage efficacy trials for NVX-CoV2373 sponsored…

On Nov. 29, 2020, Moderna announced a supply agreement with the United Kingdom (UK) government for an additional…

On Nov. 25, 2020, Moderna announced that the European Commission (EC) has approved an agreement to secure 80…

On Nov. 25, 2020, BioNTech and InstaDeep announced a multi-year strategic collaboration aimed at applying the latest advances…
On Nov. 24, 2020, Roche announced that the U.S. Food and Drug Administration (FDA) had approved a supplemental…
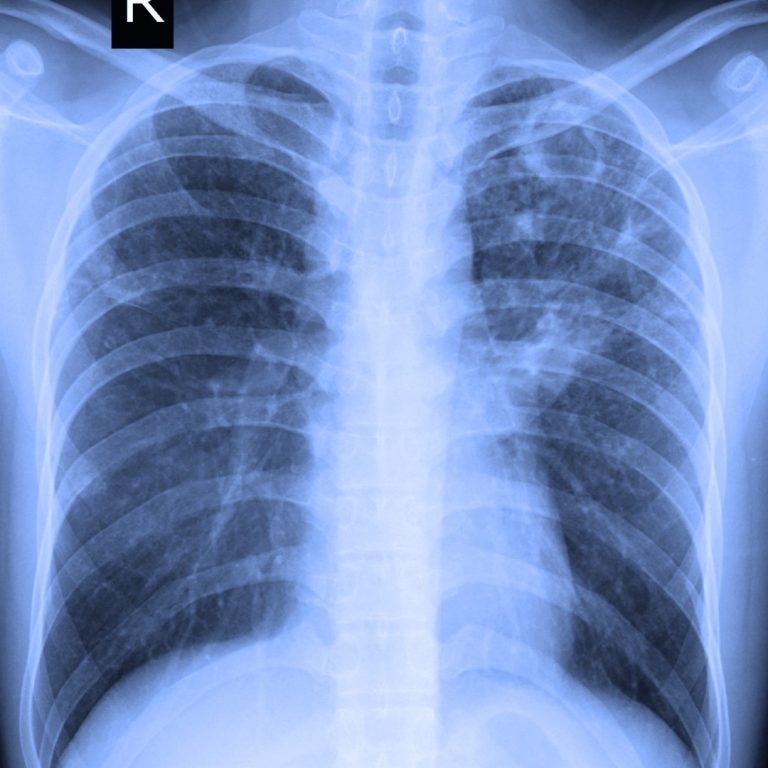
On Nov. 24, 2020, Northwestern University researchers announced they had developed a new artificial intelligence (A.I.) platform that…

On Nov. 24, 2020, published data from researchers at Mayo Clinic found that physical separation reduced the exposure…

On Nov. 23, 2020, the U.S. Food and Drug Administration (FDA) expanded the approved indication for Genentech’s Xofluza…

On Nov. 23, 2020, CytoDyn announced it had reached enrollment of 293 patients in its Phase 3 trial…