Moderna announced FDA authorization of third dose of COVID-19 vaccine for immunocompromised individuals
On Aug. 13, 2021, Moderna announced that the Food and Drug Administration (FDA) had approved an update to…
On Aug. 13, 2021, Moderna announced that the Food and Drug Administration (FDA) had approved an update to…

On Aug. 12, 2021, the U.S. Department of Veterans Affairs (VA) announced that it expanded his previous COVID-19…

On Aug. 12, 2021, Moderna announced the publication of new data on the durability of the Moderna COVID-19…

On Aug. 12, 2021, Innovation Pharma released information regarding the status of its randomized, double-blind, placebo-controlled Phase 2…
On Aug. 10, 2021, the National Institutes of Health (NIH) announced that a pilot study had begun to…

On Aug. 10, 2021, Moderna announced a Memorandum of Understanding (MoU) with the government of Canada to build…

On Aug. 9, 2021, Moderna announced that the Therapeutic Goods Administration (TGA) had granted provisional registration to the…

On Aug. 9, 2021, Inovio Pharma announced that it had received regulatory allowance for two clinical trials investigating…
On Aug. 5, 2021, Novavax announced preliminary data demonstrating that a single booster dose of its recombinant nanoparticle…

On Aug. 4, 2021, Novavax announced that it had reached an agreement with the European Commission (EC) for…

On Aug. 3, 2021, CytoDyn announced that Brazil’s regulatory authority ANVISA (Agência Nacional de Vigilância Sanitária) has approved…

On Jul. 29, 2021, Vaxart announced that it has shown for the first time in clinical trials that…

On Jul. 28, 2021, GlaxoSmithKline and Vir Biotechnology announced they had signed a Joint Procurement Agreement with the…

On Jul. 27, 2021, Oragenics announced it had entered into a licensing agreement with the National Research Council…

On Jul. 23, 2021, Moderna announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human…
On Jul. 22, 2021, Moderna announced a new supply agreement with Taiwan for 20 million doses of Moderna’s…

On Jul. 20, 2021, Moderna announced that the Ministry of Health, Labour and Welfare of Japan (MHLW) and…
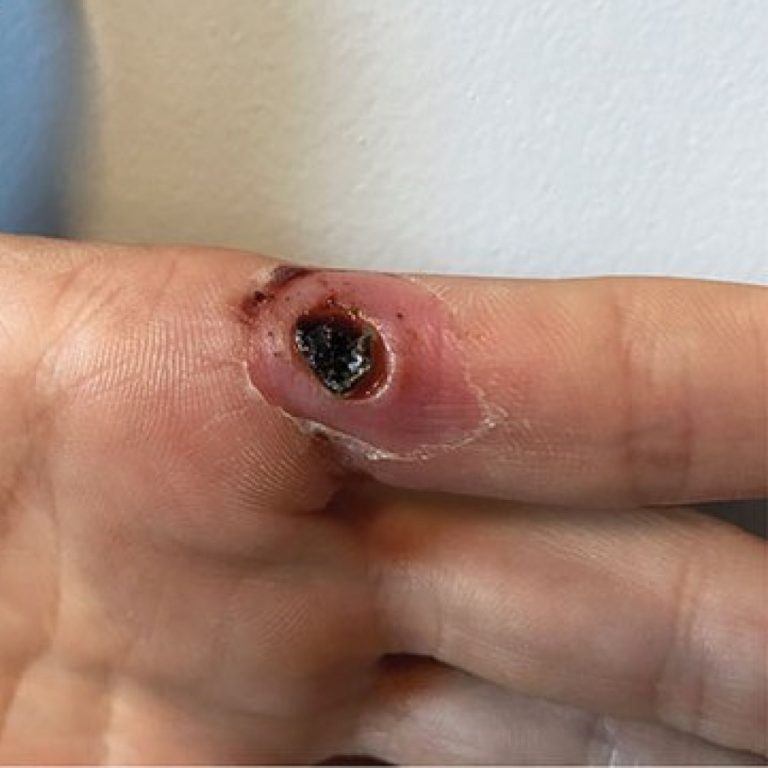
On Jul. 16, 2021, the U.S. Centers for Disease Control and Prevention (CDC) announced and the Texas Department…

On Jul. 12, 2021, Moderna announced a supply agreement with the government of Argentina for 20 million doses…

On Jul. 1, 2021, Moderna confirmed that following the European Medicines Agency’s (EMA) Committee for Human Medicines (CHMP)…

On Jun. 30, 2021, Novavax announced the publication of results from the final analysis of a pivotal Phase…

On Jun. 29, 2021, Paratek Pharmaceuticals announced it had delivered the first procurement of NUZYRA to the Biomedical…

On Jun. 29, 2021, Moderna announced new results from in vitro neutralization studies of sera from individuals vaccinated…

On Jun. 29, 2021, Moderna announced that the government of India had issued a registration certificate and a…

On Jun. 22, 2021, Moderna announced that the European Commission (EC) had purchased an additional 150 million doses…

On Jun. 21, 2021, Tonix Pharmaceuticals announced that it planned to develop TNX-102 SL (cyclobenzaprine HCl sublingual tablets)…

On Jun. 16, 2021, Moderna announced that the U.S. government had purchased an additional 200 million doses of…

On Jun. 16, 2021, Kaiser Permanente Washington Health Research Institute (KPWHRI) announced that was recruiting volunteers in the…

On Jun. 15, 2021, Moderna and Magenta Investments, an industrial conglomerate in the United Arab Emirates (UAE), announced…

On Jun. 14, 2021, University of British Columbia researchers Dr. Jayachandran Kizhakkedathu, Dr. Caigan Du and Dr. Christopher…