Vaccine breakthrough means no more chasing strains
On Apr. 15, 2024, scientists at University of California Riverside demonstrated a new, RNA-based vaccine strategy that is…

On Apr. 15, 2024, scientists at University of California Riverside demonstrated a new, RNA-based vaccine strategy that is…

On Apr. 12, 2024, the U.S. Centers for Disease Control and Prevention (CDC) announced that Vietnam had reported…
On Apr. 12, 2024, Oregon Health Sciences University (OHSU) announced research that shows using live SARS-CoV-2 virus revealed…
On Apr. 10, 2024, LabCorp announced that the U.S. Food and Drug Administration (FDA) had granted Emergency Use…

On Apr. 5, 2024, the U.S. Centers for Disease Control and Prevention (CDC) announced that Texas had reported…

On Apr. 2, 2024, the U.S. Department of Agriculture (USDA) confirmed the detection of highly pathogenic avian influenza…

On Apr. 1, 2024, the U.S. Department of Agriculture (USDA) confirmed the detection of highly pathogenic avian influenza…

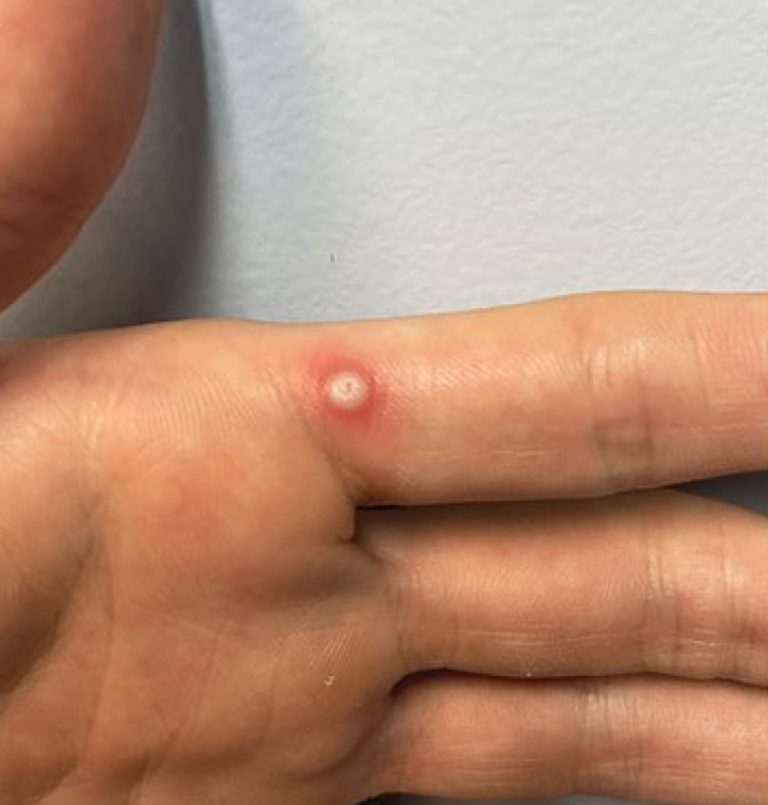
On Mar. 29, 2024, the U.S. Centers for Disease Control and Prevention (CDC) reported the first U.S. human…

On Mar. 28, 2024, the Idaho State Department of Agriculture (ISDA) announced it had identified a highly pathogenic…

On Mar. 26, 2024, Roche announced that the U.S. Food and Drug Administration (FDA) had approved the cobas…

On Mar. 25, 2024, immediately following the first detection of H5N1 in dairy cattle in the Texas panhandle…

On Mar. 22, 2024, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization for Pemgarda…

On Mar. 20, 2024, the Minnesota Board of Health announced that a Stevens County goat kid (juvenile goat)…
On Mar. 14, 2024, the Institute for Health Metrics and Evaluation (IHME) released all-cause mortality, life expectancy, and…

On Mar. 8, 2024, the U.S. Food and Drug Administration’s (FDA) Vaccines and Related Biological Products Advisory Committee…
On Mar. 1, 2024, researchers at the National Institutes of Health (NIH) announced they had identified antibodies targeting…

On Feb. 29, 2024, Valneva announced that the U.S. Centers for Disease Control and Preventionメs (CDC) Advisory Committee…

On Feb. 28, 2024, the U.S. Centers for Disease Control and Preventionメs (CDC) Advisory Committee on Immunization Practices…

On Feb. 20, 2024, the U.S. Department of Agriculture (USDA) confirmed the presence of highly pathogenic avian influenza…

On Feb. 12, 2024, the U.S. Centers for Disease Control and Prevention (CDC) announced that Cambodia had reported…

On Feb. 9, 2024, the state of Alaska Department of Health Alaska Department of Health confirmed the first…

On Feb. 8, 2024, Deschutes County Health Services in Bend, Oregon confirmed a case of human plague in…
On Feb. 6, 2024, Oregon Health & Science University (OHSU) announced research that revealed as much as a…
On Feb. 5, 2024, CSL and and Arcturus Therapeutics announced the results of a follow-up analysis of a…

On Jan. 31, 2024, a U.S. Centers for Disease Control and Prevention (CDC) co-authored study published in Influenza…

On Jan. 27, 2024, the National Health Commission of the People’s Republic of China notified the World Health…

On Jan. 22, 2024, the University of California, Davis announced the first reported outbreak of high pathogenicity H5N1…

On Jan. 9, 2024, biologists in Alaska have confirmed the first case of a polar bear dying after…

On Jan. 4, 2024, France announced it had detected bird flu on a duck farm in the Vendee…

On Jan. 4, 2024, AstraZeneca Sanofi’s Beyfortus (nirsevimab), a long-acting monoclonal antibody, was approved in China for the…