WHO announced Recommendations for influenza vaccine for the 2025 southern hemisphere influenza season
On Sept. 27, 2024, the World Health Organization (WHO) announced the recommendations for the viral composition of influenza…

On Sept. 27, 2024, the World Health Organization (WHO) announced the recommendations for the viral composition of influenza…

On Sept. 25, 2024, drug-resistant pathogens could jeopardise the food supply of over two billion people and increase…
On Sept. 25, 2024, U.S. healthcare workers receiving additional vaccine doses during the Omicron period (December 2021 to…

On Sept. 24, 2024, the Mark O. Hatfield Clinical Research Center at the National Institutes of Health (NIH)…
On Sept. 24, 2024, U.S. President Joe Biden announced the donation of 1 million mpox vaccine doses and…
On Sept. 19, 2024, Oxitec announced it had launched Sparks™, a new commercial platform to accelerate the global…
On Sept. 19, 2024, the nonprofit organization Focus on Lyme and Aces Diagnostics announced a breakthrough in Lyme…

On Sept. 19, 2024, the European Medical Agency (EMA) announced it had recommended extending the indication of the…

On Sept. 19, 2024, a U.S. population-based birth cohort study was released that showed that 53.4% of babies…

On Sept. 19, 2024, an international team of researchers announced a study that genetically traced the epicenter of…
On Sept. 19, 2024, the World Health Organization (WHO) announced that Jordan had become the first country in…

On Sept. 17, 2024, researchers from Tufts University announced they had received a $20.7 million grant from the…
On Sept. 16, 2024, A 24-year-old student has died from the Nipah virus in the southern Indian state…

On Sep. 13. 2024, Novavax announced that doses of the Novavax COVID-19 Vaccine, Adjuvanted (2024-2025 Formula) (NVX-CoV2705) to…

On Sept. 13, 2024, CSL Seqirus and sa-mRNA pioneer Arcturus Therapeutics announced that Japan’s Ministry of Health, Labor…

On Sept. 12, 2024, the Finnish Institute for Health and Welfare (THL) reported that Poliovirus had been found…

On Sept. 11, 2024, an international team of scientists led by the Leibniz Institute on Aging announced they…

On Sep. 2, 2024, the World Health Organization (WHO) confirmed that a laboratory-confirmed case of human infection with…

On Aug. 30, 2024, Novavax announced the Novavax COVID-19 Vaccine, Adjuvanted (2024-2025 Formula) (NVX-CoV2705) had received Emergency Use…
On Aug. 29, 2024, researchers at the Garvan Institute of Medical Research announced they had mapped 50,000 of…
On Aug. 29, 2024, a small team of scientists has launched an open-source database for some of the…

On Aug. 28, 2024, survey data from the Annenberg Public Policy Center (APPC) found that the number of…

On Aug. 28, 2024, Northwestern University and University of Texas-Southwestern announced results of a study that found bacterial…

On Aug. 27, 2024, the New Hampshire Department of Health and Human Services (DHHS) identified an adult who…
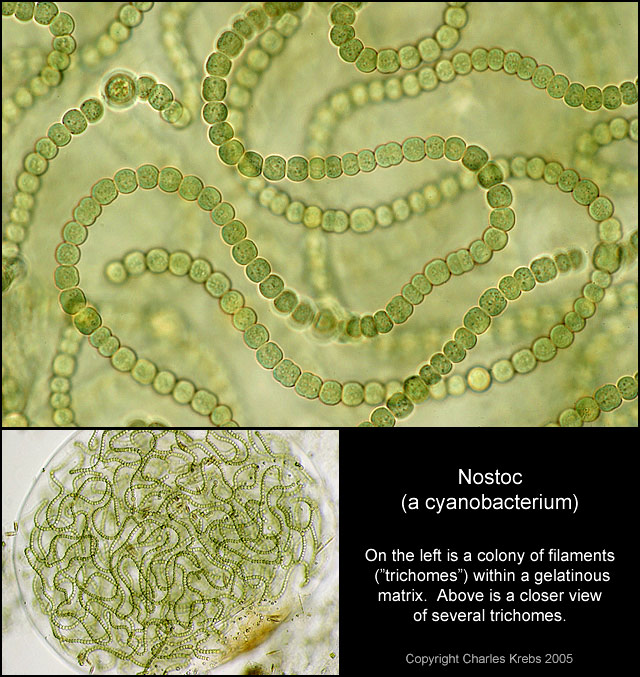
On Aug. 21, 2024, a research team at the University of Texas at Austin reported that the first…

On Aug. 16, 2024, the U.S. Centers for Disease Control and Prevention (CDC) reported that COVID-19 infections are…

On Aug. 8, the World Health Organization (WHO) announced results of a study that showed the introduction of…

On Aug. 6, 2024, a team of researchers, led by the University of Houston, announced they had discovered…

On Aug. 6, 2024, the University of Pennsylvania announced it had sued German biotechnology firm BioNTech n Pennsylvania…

On Jul. 26, 2024, the Maryland Department of Health advised consumers not to eat certain deli meats produced…