U.S. Implements New Export Controls to Address National Security Risks Related to Biotechnology
On Jan. 15, 2025, the U.S. Department of Commerce’s Bureau of Industry and Security (BIS) released an Interim…

On Jan. 15, 2025, the U.S. Department of Commerce’s Bureau of Industry and Security (BIS) released an Interim…

On Jan. 15, 2025, the California Department of Public Health (CDPH) reported thatqOne confirmed human infection with influenza…
On Jan. 14, 2025, The U.S. Department of Defense (DoD) has awarded up to $11.9 million to Oregon…

On Jan. 13, 2025, the Texas Animal Health Commission (TAHC) and the United States Department of Agriculture’s (USDA)…

On Jan. 13, 2025, the Los Angeles County Department of Public Health (LACDPH) reported that five indoor-only cats…

On Jan. 13, 2025, the World Health Organization (WHO) informed its Member States and IHR State Parties of…

On Jan. 13, 2025, scientists from the Francis Crick Institute, UCL, UCLH and Personalis announced they have found…

On Jan. 10, 2025, a study of chronic wasting disease (CDW), the prion disease that causes deer, elk…
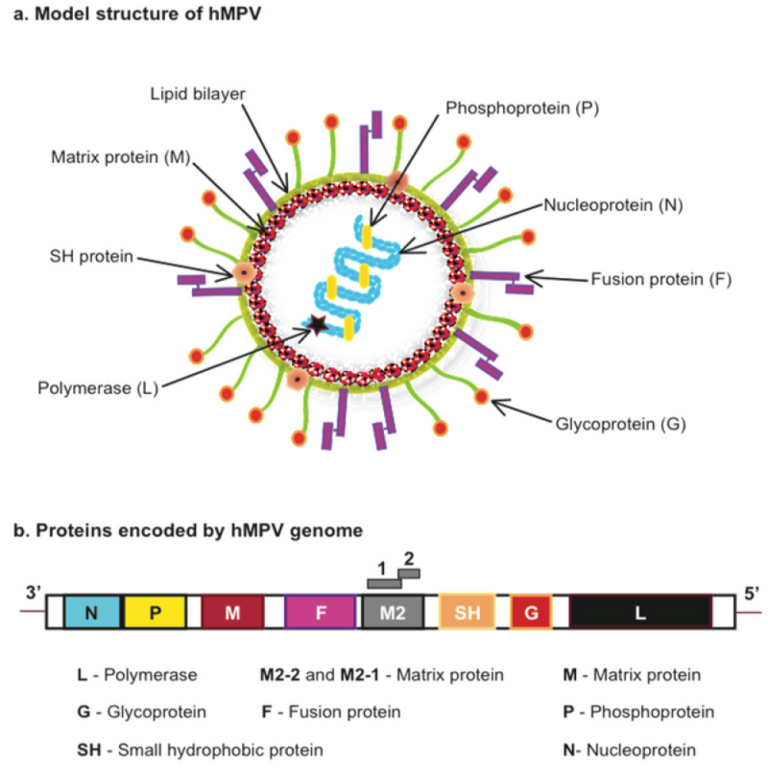
On Jan. 7, 2025, the World Health Organization (WHO) reported that trends in acute respiratory infections (ARI) in…
On Jan. 7, 2025, scientists at The University of Queensland (UQ) and the University of Sydney announced they…

On Jan. 7, 2025, the U.S. Army Public Health Command Europe reported that cases of norovirus, a wretched…
On Jan. 6, 2025, the Louisiana Department of Health reports the patient who had been hospitalized with the…

On Jan. 3, 2025, the California Department of Public Health (CDPH) reported that Whooping cough (pertussis) activity in…

On Jan. 3, 2025, the Centers for Disease Control and Prevention (CDC) reported that during Christmas week, respiratory…

On Jan. 3, 2025, the U.S. Department of Health and Human Services (HHS) announced it would award $306…

On Jan. 2, 2025, SIGA Technologies announced that its antiviral treatment TEPOXX (tecovirimat 200 mg capsules), marketed as…
On Jan. 2, 2025, the U.S. Centers for Disease Control and Prevention (CDC) reported an increased incidence of…
On Dec. 31, 2024, the U.S. Centers for Disease Control and Prevention released a study of the largest…
On Dec. 31, 2024, a study was published of patients with kidney disease who are at high risk…
On Dec. 30, 2024, the Oregon Health Authority reported it had registered 1,105 cases of pertussis—also known as…

On Dec. 30, 2024, the Maricopa County Department of Public Health (MCDPH), as part of routine wastewater surveillance…

On Dec. 30, 2024, a study published in JAMA Network Open found that the estimated effectiveness of at least…
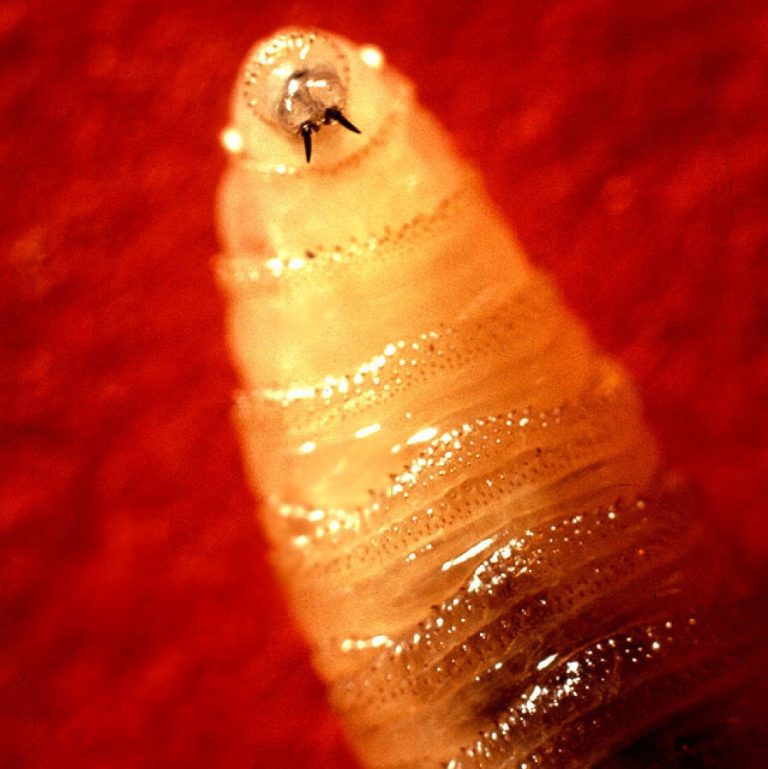
On Dec. 30, 2024, Texas Parks and Wildlife Department (TPWD) asked hunters and other outdoor enthusiasts in South…
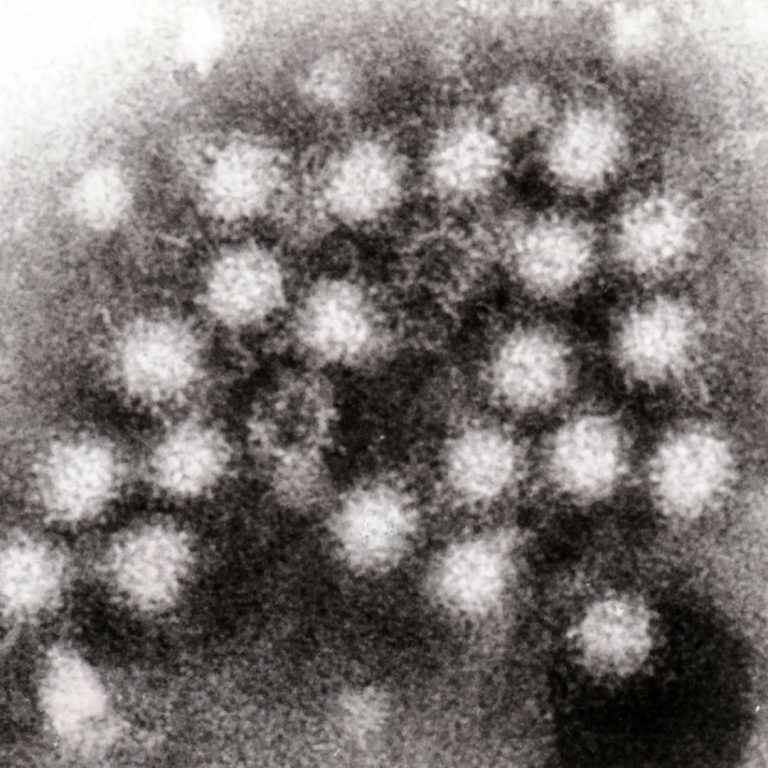
On Dec. 28, 2024, the U.S. Centers for Disease Control and Prevention (CDC) reported that cases of a…

On Dec. 27, 2024, BioNTech announced it had entered into two separate settlement agreements with the U.S. National…

On Dec. 26, 2024, the Oregon Department of Agriculture (ODA) announced it is alerting pet owners that samples…

On Dec. 24, 2024, the World Health Organization (WHO) announced that SARS-CoV-2, the virus that causes COVID-19, largely…

On Dec. 24, 2024, the Minnesota Department of Natural Resources (MNDNR) is investigating a number of wild waterfowl…

On Dec. 23, 2024, an Annenberg Public Policy Center (APPC) health survey conducted in November 2024 showed that…

On Dec. 23, 2024, an international team of researchers reported results of a study that showed the escalating…