Moderna received U.S. FDA approval for updated COVID-19 vaccine
On Sept. 11, 2023, Moderna announced the U.S. Food and Drug Administration (FDA) had approved the supplemental Biologics…

On Sept. 11, 2023, Moderna announced the U.S. Food and Drug Administration (FDA) had approved the supplemental Biologics…

On Sept. 11, 2023, Pfizer and BioNTech announced that the U.S. Food and Drug Administration (FDA) approved the…

On Sept. 6, 2023, Moderna announced that clinical trial data from its research assay confirmed its updated COVID-19…

On Aug. 31, 2023, the U.S. Food and Drug Administration (FDA) cleared for marketing the updated 23andMe Personal…

On Aug. 30, 2023, Pfizer and BioNTech announced that the Committee for Medicinal Products for Human Use (CHMP)…
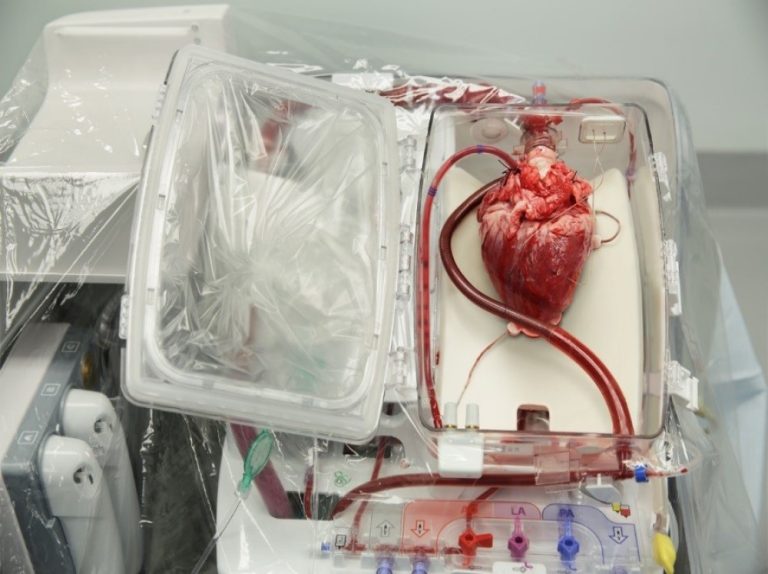
On Aug. 22, 2023, a study led by researchers at Washington University School of Medicine in St. Louis…

On Aug. 21, 2023, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved ABRYSVO (Respiratory…

On Aug. 4, 2023, Biogen and Sage Therapeutics announced that the U.S. Food and Drug Administration (FDA) had…

On Aug. 3, 2023, Merck announced that the U.S. Food and Drug Administration (FDA) had approved an expanded…
On Jul. 31, 2023, Emergent BioSolutions announced that it was awarded a 10-year contract by the Biomedical Advanced…
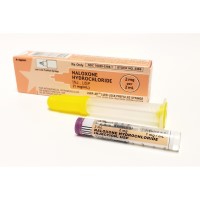
On Jul. 28, 2023, the U.S. FDA approved RiVive, 3 milligram (mg) naloxone hydrochloride nasal spray for over-the-counter…
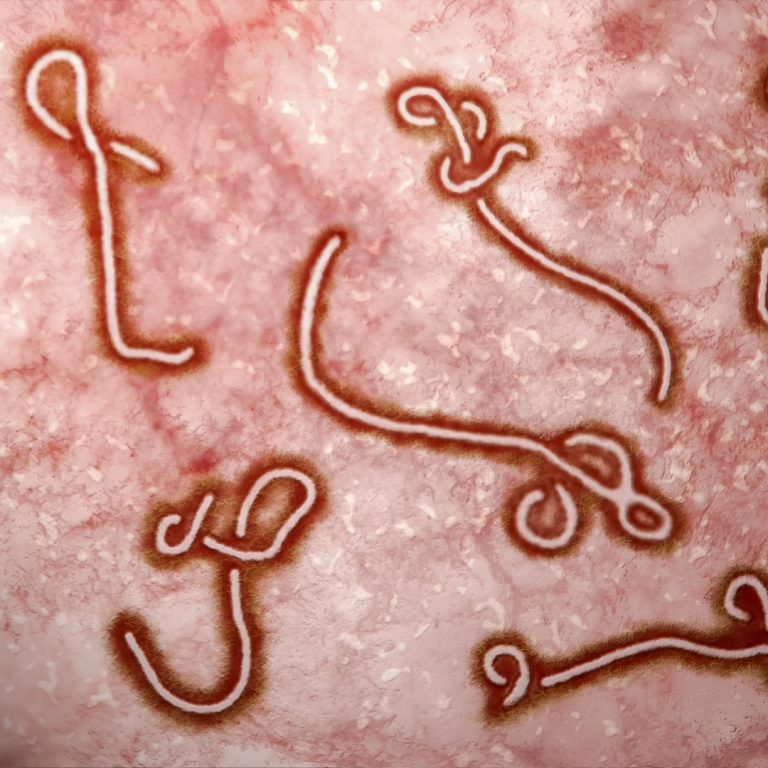
On Jul. 28, 2023, the U.S. Food and Drug Administration (FDA) approved Merck’s Ervebo, a vaccine for the…

On Jul. 20, 2023, Emergent BioSolutions announced that the U.S. Food and Drug Administration (FDA) had approved CYFENDUSル…

On Jul. 17, 2023, the FDA approved AstraZeneca’s Beyfortus (nirsevimab-alip) for the prevention of Respiratory Syncytial Virus (RSV)…

On Jul. 14, 2023, Gilead Sciences announced that the U.S. Food and Drug Administration (FDA) had approved a…

On Jul. 6, 2023, Eisai and Biogen announced that the U.S. Food and Drug Administration (FDA) had approved the…

On Jun. 29, 2023, the U.S. Food and Drug Administration approved Roctavian, an adeno-associated virus vector-based gene therapy…

On Jun. 22, 2023 AquaBounty Technologies announced that the Company will pause the construction of its farm in…

On Jun. 22, 2023, the U.S. Food and Drug Administration (FDA) approved Sarepta Therapeutics’s Elevidys, the first gene…

On Jun. 20, 2023, the U.S. Food and Drug Administration (FDA) approved Boehringer Ingelheim’s Jardiance (empagliflozin) and Synjardy…

On Jun. 16, 2023, the U.S. Food and Drug Administration’s (FDA) Vaccines and Related Biological Products Advisory Committee…
On Jun. 6, 2023, the U.S. Food and Drug Administration (FDA) granted granted marketing authorization for the Cue COVID-19…
On May 31, 2023, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved ABRYSVO (Respiratory…

On May 25, 2023, the U.S. Food and Drug Administration (FDA) approved the oral antiviral Paxlovid (nirmatrelvir tablets…

On May 24, 2023, the U.S. FDA approved Braeburn pharmaceutical’s Brixadi (buprenorphine) extended-release injection for subcutaneous use (under…

On May 19, 2023, the U.S. Food and Drug Administration (FDA) cleared the Beta Bionics iLet ACE Pump…

On May 18, 2023, the U.S. FDA approved approved Rinvoq (upadacitinib) for adults with moderately to severely active…

On May 11, 2023, the U.S. Food and Drug Administration (FDA) announced the supplemental approval of Otsuka Pharmaceutical…

On May 3, 2023, Washington State University (WSU) made history in the United States (US) by becoming the…

On May 3, 2023, Vertex announced the Food and Drug Administration (FDA) ha approved KALYDECOᆴ (ivacaftor) for use…