The Best Pharmaceuticals for Children Act improved safety and efficacy of patented and off-patent medicines for children
In 2002, the U.S. Congress passed the Best Pharmaceuticals for Children Act that improved safety and efficacy of…

In 2002, the U.S. Congress passed the Best Pharmaceuticals for Children Act that improved safety and efficacy of…

On Dec. 17, 2001, the acquisition of Immunex by Amgen was announced for $16 billion in stock and…
On Sept. 13, 2001, the Food and Drug Administration (FDA) announced it had approved Genentech’s request for it’s…
On May 10, 2001, the U.S. Food and Drug Administration (FDA) approved Gleevec, the world’s first targeted cancer…
On Mar. 29, 2001, the Food and Drug Administration (FDA) announced it had approved Genentech’s drug Valcyte (valganciclovir…

On Jan. 12, 2001, Corus Pharma was incorporated as a private venture-backed development stage biopharmaceutical company focused on…

In 2001, SynCardia Systems, a private company formed to commercialize the Jarvik 7 artificial heart, was founded by…

On Sept. 22, 2000, Pathogenesis was acquired by Emeryville, California-based Chiron for $660 million or $38.50 per share….

On Mar. 21, 2000, the Supreme Court ruled 5 to 4 along ideological lines, that the U.S. Food…

In 2000, Eli Lilly and Takeda Chemical launched Actos, an oral anti diabetes agent. In 2005, a PROactive…

On Dec. 29, 1999, the U.S. Food and Drug Administration (FDA) approved Bexarotene (Targretin) to treat cutaneous T-cell…

On Dec. 9, 1999, the diphtheria and tetanus toxoids and acellular pertussis vaccine (Tripedia by Connaught) was licensed….
On Nov. 15, 1999, the FDA approved LASIK corrective eye surgery. LASIK stands for Laser-Assisted In Situ Keratomileusis…

On Aug. 29, 1999, Merck, West Point, Pennsylvania) received approval from the FDA of a supplement to Merck’s…

On Jul. 11, 1999, Daiichi Sankyo and Eli Lilly announced the FDA approved Effientル(prasugrel) tablets for the reduction…

On Mar. 27, 1999, Novo Nordisk, the ZymoGenetics parent company, announced approval of NovoSeven by the Food and…

On Mar. 23, 1999, the Food and Drug Administration (FDA) announced a rule publication that defined dietary supplements…
On Mar. 17, 1999, the Hybrid Capture II human papillomavirus (HPV) DNA test was approved by the U.S….
On Mar. 17, 1999, the Food and Drug Administration (FDA) published a regulation mandating that labels of all…

On Jan. 17, 1999, Jane E. Henney, M.D., appointed by President Bill Clinton, became the first woman to…

On Jan. 15. 1999, the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices…
On Jan. 1, 1999, HIV infection in adults (clients 13 years of age or older) became reportable by…
On Sept. 25, 1998, the U.S. Food and Drug Administration (FDA) approved the monoclonal antibody Herceptin (Trastuzumab) for…

On Aug. 31, 1998, Rotashield (Wyeth Laboratories, Marietta, Pennsylvania) was licensed by the U.S. Food and Drug Administration…
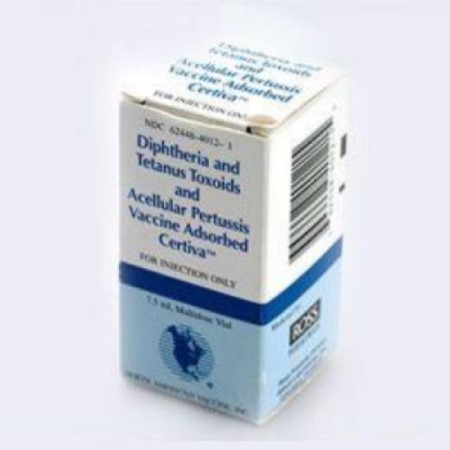
On Jul. 29, 1998, the diphtheria and tetanus toxoids and acellular pertussis vaccine adsorbed (Certiva by North American…

On April 30, 1998, the U.S. Food and Drug Administration (FDA) approved the Genentech’s drug Xeloda (capecitabine) for…

On Mar. 27, 1998. the Pfizer drug Viagra (Sildenafil) was approved by the FDA to treat erectile dysfunction….

In 1998, the first phase to consolidate U.S. Food and Drug Administration (FDA) laboratories nationwide from 19 facilities…

In 1998, the Pediatric Rule was implemented that required drug manufacturers to study the efficacy and safety of…

On Nov. 10, 1998, Immunex announced that ENBREL(tm) (etanercept) had received approval from the U.S. Food and Drug…