The FDA informed public about possible accuracy concerns with Abbott ID NOW point-of-care test
On May 14, 2020, the FDA alerted the public to early data that suggest potential inaccurate results from…

On May 14, 2020, the FDA alerted the public to early data that suggest potential inaccurate results from…

On May 14, 2020, AIM ImmunoTech announced the FDA authorized the first human trial assessing the safety and…

On May 13, 2020, Royal Philips announced that it had received 510(k) clearance from the FDA to market…

On May 13, 2020, Thermo Fisher Scientific announced it will expand its response to the COVID-19 pandemic by…

On May 12, 2020, Abbott announced the FDA issued an Emergency Use Authorization (EUA) for the company’s molecular…

On May 12, 2020, Moderna announced the U.S. Food and Drug Administration (FDA had granted Fast Track designation…

On May 12, 2020, Thermo Fisher Scientific announced the FDA has further expanded emergency use authorization (EUA) for…

On May 11, 2020, Todos Medical announced an exclusive distribution agreement with Gnomegen for the distribution of its…

On May 11, 2020, the FDA announced actions to accelerate the development of prevention and treatment options for…

On May 11, 2020, Abbott announced the FDA issued an Emergency Use Authorization (EUA) for the company’s SARS-CoV-2…

On May 11, 2020, Bellerophon Therapeutics announced the FDA accepted its Investigational New Drug (IND) application, allowing the…

On May 8, 2020, the U.S. Food and Drug Administration (FDA) issued the first emergency use authorization (EUA)…

On May 8, 2020, the FDA issued an emergency use authorization (EUA) to Rutgers Clinical Genomics Laboratory for…

On May 8, 2020, RedHill Biopharma announced the U.S. Food and Drug Administration (FDA) had approved its Investigational…

On May 8, 2020, Quidel announced it had received Emergency Use Authorization (EUA) from the FDA to market…

On May 7, 2020, OPTI Medical Systems, an IDEXX Laboratories subsidiary announced the U.S. Food and Drug Administration…

On May 6, 2020, PharmaCyte Biotech announced it had received Medical Devices Establishment Registration with the FDAメs Center…

On May 6, 2020, the U.S. Food and Drug Administration (FDA) approved Tabrecta (capmatinib) for the treatment of…

On May 5, 2020, PerkinElmer announced the FDA provided Emergency Use Authorization (EUA) for EUROIMMUN’s (a PerkinElmer company)…

On May 5, 2020, Organicell Regenerative Medicine announced the FDA has approved the Investigational New Drug application for…

On May 5, 2020, Diffusion Pharmaceuticals announced the FDA accelerated its review of the Companyメs clinical development plan…

On May 4, 2020, bioMerieux announced that BioFire Diagnostics, its subsidiary specialized in syndromic infectious disease testing, has…

On May 4, 2020, Vir Biotech and Alnylam Pharma announced the selection of a development candidate for VIR-2703,…

On May 4, 2020, Cerus announced FDA regulatory approval for manufacture of INTERCEPT plasma with a new, alternative…

On May 4, 2020, DNA Genotek, a subsidiary of OraSure Technologies, announced that its ORAcollect RNA kit (OR-100)…

On May 2, 2020, Roche announced that the FDA has issued an Emergency Use Authorization for its new…
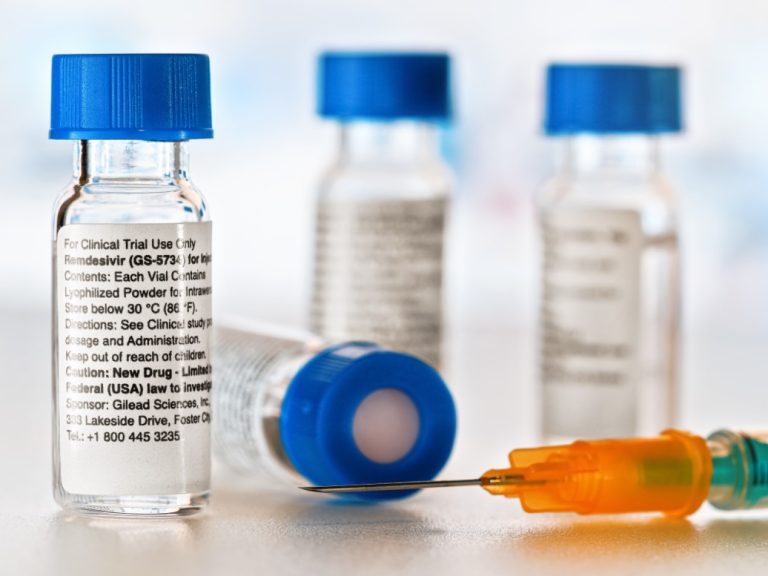
On May 1, 2020, Gilead announced the FDA granted emergency use authorization (EUA) for the investigational antiviral remdesivir…

On May 1, 2020, the FDA issued an Emergency Use Authorization (EUA) for the investigational antiviral drug remdesivir…

On May 1, 2020, Janssen Pharma, a Johnson & Johnson company, announced the U.S. Food and Drug Administration…

On Apr. 30, 2020, the FDA included, under the ventilator emergency use authorization (EUA), a ventilator developed by…