US EPA moves to take action on review of fluoride in drinking water
On Jan. 28, 2026, the U.S. Environmental Protection Agency (EPA) launched the first step of its expedited review…
On Jan. 28, 2026, the U.S. Environmental Protection Agency (EPA) launched the first step of its expedited review…

On Jan. 28, 2026, researchers at Google DeepMind have unveiled their latest artificial intelligence tool and claimed it…
On Jan. 27, 2026, a University of Cambridge study shows menopause is linked to reductions in grey matter…
On Jan. 21, 2026, Rice University launched the Global Brain Economy Initiative (GBEI) during the annual meeting of…
On Jan. 20, 2026, researchers announced they have identified two biomarkers — verbal learning and identifying other peoples’…

On Jan. 13, 2026, The American Cancer Society (ACS) released Cancer Statistics, 2026, the organization’s annual report on cancer facts and trends. The findings show, for…
On Jan. 9, 2026, scientists at the Icahn School of Medicine at Mount Sinai, in partnership with the Multiple Myeloma Research…

On Jan. 8, 2026, Chinese drug makers’ licensing deals more than doubled in 2025 from a year earlier…

On Jan. 8, 2026, a major advance in cell biology has revealed how our cells safeguard their genetic…
On Jan. 5, 2026, the Acting Director of the Centers for Disease Control and Prevention signed a decision…
On Jan. 5, 2026, researchers at the University of Illinois Urbana-Champaign have developed a groundbreaking new tool that…

On Jan. 5, 2026, Novo Nordisk announced that the Wegovy® pill is now available, providing those seeking help…

On Jan. 1, 2026, the Centers for Disease Control and Prevention (CDC) reported that in September 2024, the…

On Dec. 22, 2025, Novo Nordisk announced announced that the US Food and Drug Administration (FDA) has approved…

On Dec. 18, 2025, new national and state data was released by the Partnership to Fight Chronic Disease…
On Dec. 18, 2025, Novo Nordisk announced the submission of a New Drug Application (NDA) to the U.S….

On Dec. 18, 2025, scientists at the Hebrew University of Jerusalem revealed that bacteriophages use a small RNA…
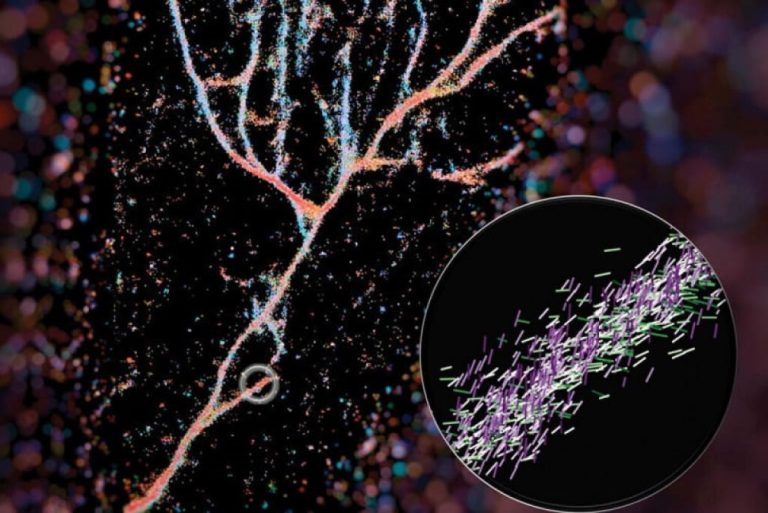
On Dec. 17, 2025, A survey of Alzheimer’s disease prevalence in Norway confirms earlier estimates and might show…
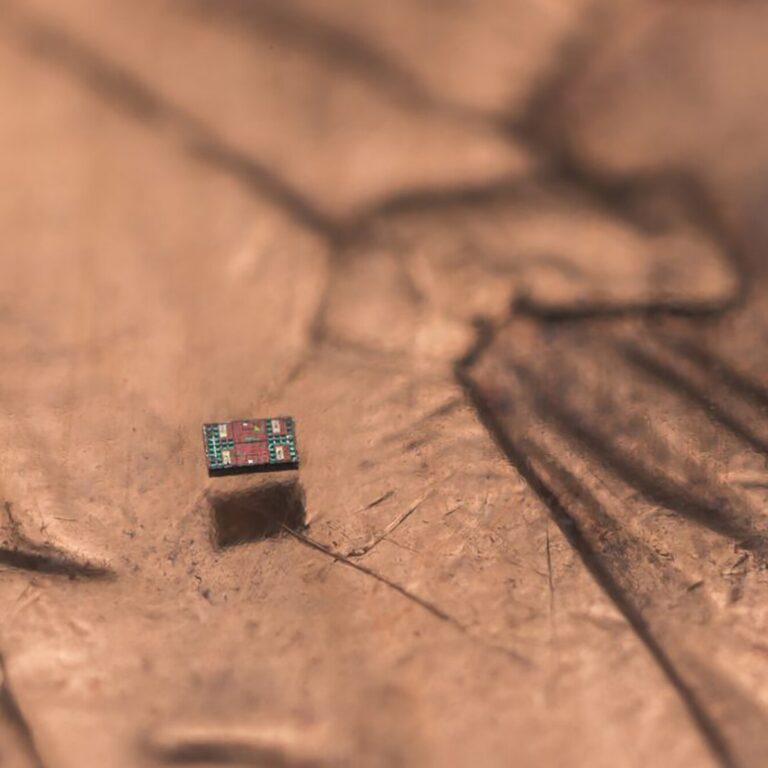
On Dec. 17, 2025, researchers at the University of Pennsylvania’s (UPenn) School of Engineering and Applied Science, and…
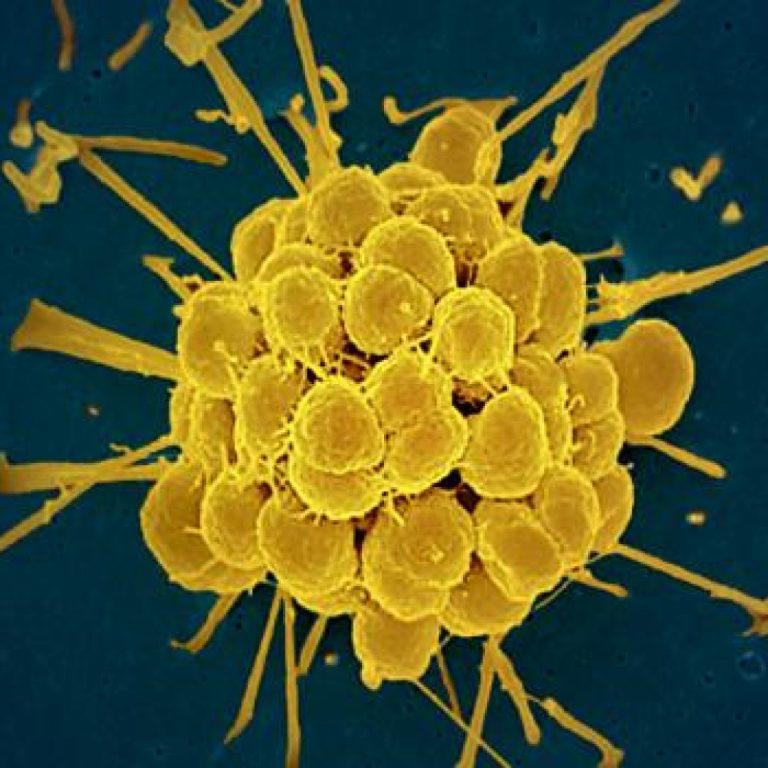
On Dec. 12, 2025, the U.S. Food and Drug Administration (FDA) announced it has approved two new oral…

On Dec. 11, 2025, the California Institute for Regenerative Medicine (CIRM) governing board approved funding of over $160…

On Dec. 11, 2025, a new analysis from a World Health Organization(WHO) global expert committee on vaccine safety…
On Dec. 10, 2025, researchers from Gladstone Institutes and Stanford University announced they have leveraged a comprehensive method…

On Dec. 5, 2025, a federal vaccine advisory committee voted on Friday to end the longstanding recommendation that…
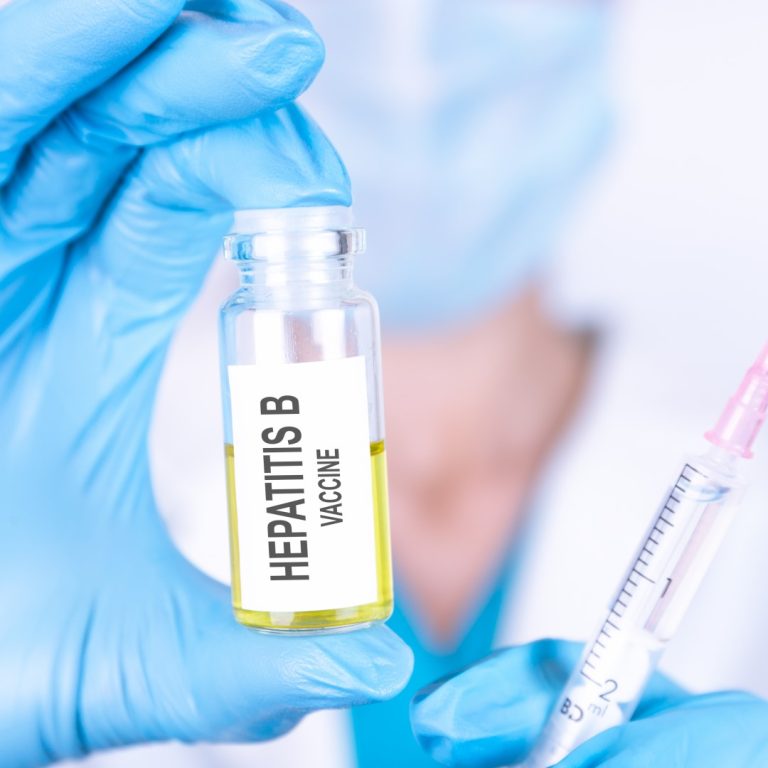
On Dec. 5, 2025, the West Coast Health Alliance (WCHA) announced that it strongly supports that hepatitis B…

Dec. 4, 2025, new research from the University of Washington (UW) underscores risks of using publicly available large-language…

On Dec. 3, 2025, Massachusetts Institute of Technology (MIT) engineers announced they can accurately measure blood glucose by…

On Dec. 2, 2025, findings in the latest Global Burden of Disease 2023 study published in The Lancet…

On Dec. 1, 2025, the World Health Organization (WHO) has released its first guideline on the use of…

On Dec. 1, 2025, in an unusual act of philanthropy, an anonymous donor has committed more than $50…