Emerald Organic and Todos Medicalfor JV to supply USwith COVID-19 rapid point-of-care testing kits
On Mar. 24, 2020, Emerald Organic Products and Todos Medical announced the creation of Corona Diagnostics, LLC, a…

On Mar. 24, 2020, Emerald Organic Products and Todos Medical announced the creation of Corona Diagnostics, LLC, a…

On Mar. 24, 2020, Partner Therapeutics that Leukine (sargramostim, rhu-GM-CSF) is being assessed in the SARPAC trial (sargramostim…

On Mar. 24, 2020, the Andrew and Corey Morris-Singer Foundation donated $1.6 million to OHSU to support the…

On Mar. 24, 2020, T2 Biosystems announced it had entered into a worldwide licensing agreement for a rapid…

On Mar. 24, 2020, Windtree Therapeutics announced it will study its proprietary KL4 surfactant to potentially mitigate the…
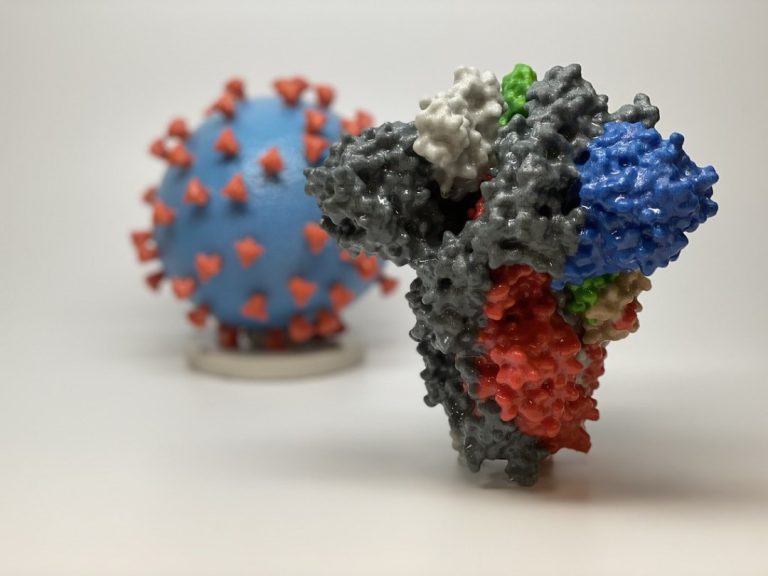
On Mar. 24, 2020, to help in educating the public and in the hope of better conveying the…

On Mar. 23, 2020, Resverlogix announced that it was seeking to collaborate with organizations currently testing therapies for…

On Mar. 23, 2020, the National Institutes of Health (NIH) announced that it had launched an important online…

On Mar. 23, 2020, researchers from the University of Oxford the launch of a new clinical trial to…
On Mar. 23, 2020, the UK Government announced funding of £20 million to support clinicians and scientists in…

On Mar. 23, 2020, the Amgen Foundation announced an initial commitment of up to $12.5 million to support…

On Mar. 23, 2020, Sorrento and SmartPharm Therapeutics announced a research and development collaboration to develop a next-generation,…

On Mar. 23, 2020, Drug Innovation Ventures at Emory (DRIVE), and Ridgeback Biotherapeutics announced a collaboration to rapidly…

On Mar. 23, 2020, Covetrus reported that the company saw strong demand in its prescription management and online…

On Mar. 23, 2020, researchers at Washington University School of Medicine in St. Louis announced they were investigating…

On Mar. 23, 2020, Heat Biologics announced that it was collaborating with the University of Miami to develop…

On Mar. 23, 2020, LabCorpᆴ announced that it is exploring all options to prioritize COVID-19 testing for the…

On Mar. 23, 2020, BioReference Labs, an OPKO Health company, announced a collaboration with the City of Detroit…
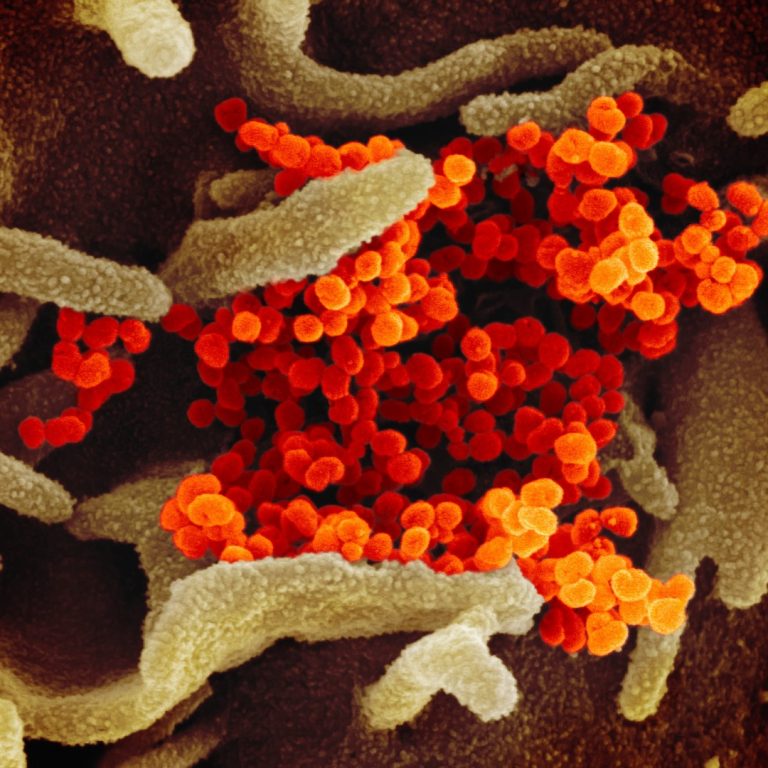
On Mar. 23, 2020, Soligenix announced that its ongoing collaboration with the University of Hawaii at Manoa (UH…
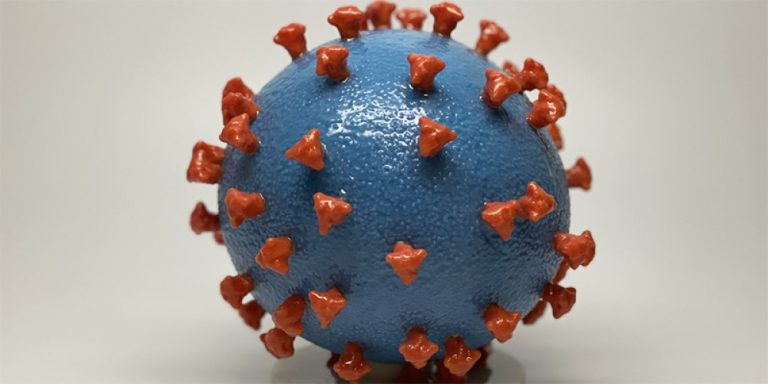
On Mar. 23, 2020, CSL announced that it was exploring development of a hyperimmune serum that could be…

On Mar. 21, 2020, the FDA issued the first emergency use authorization for a point-of-care COVID-19 diagnostic for…

On Mar. 21, 2020, through its “Peace of Mind” program, Quest Diagnostics took extra measures to ensure individuals…
On Mar. 20, 2020, Cardax released a white paper outlining the potential role of astaxanthin in the treatment…

On Mar. 20, 2020, PRA Health Sciences announced the commercial availability of the COVID-19 Monitoring Program, a mobile…
On Mar. 20, 2020, Bellerophon Therapeutics announced the U.S. Food and Drug Administration (FDA) had granted emergency expanded…

On Mar. 20, 2020, Amneal Pharma announced that it was responding to the national COVID-19 health emergency by…

On Mar. 20, 2020, Chembio Diagnostics announced receipt of a $4 million order from Bio-Manguinhos for the purchase…

On Mar. 20, 2020, the University of Pennsylvania announced that Pavilion project construction would be allowed to continue…

On Mar. 20, 2020, GM and Ventec Life Systems announced they were collaborating to enable Ventec to increase…

On Mar. 20, 2020, to further reduce the risk of transmission of COVID-19 and enhance the safety of…