BD announced second FDA Emergency Use Authorization, CE Mark for new COVID-19 molecular diagnostic for global use
On Apr. 13, 2020, BD (Becton, Dickinson) announced the U.S. Food and Drug Administration (FDA) had granted Emergency…

On Apr. 13, 2020, BD (Becton, Dickinson) announced the U.S. Food and Drug Administration (FDA) had granted Emergency…

On Apr. 10, 2020, BenevolentAI announced that baricitinib, which it had identified as a potential treatment for COVID-19,…
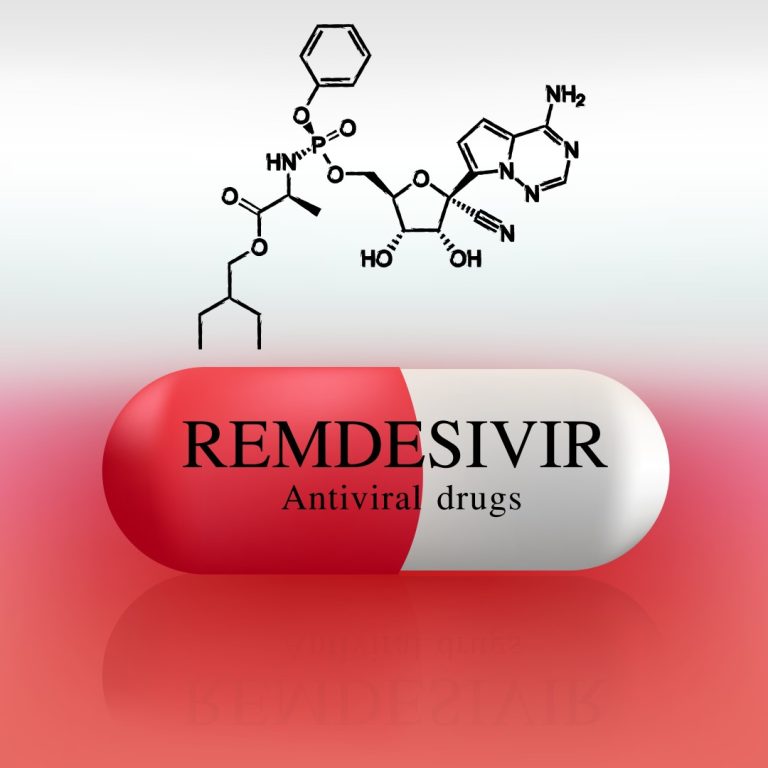
On Apr. 10, 2020, Gilead Sciences announced results from a cohort analysis of 53 patients hospitalized with severe…

On Apr. 10, 2020, Terumo BCT and Marker Therapeutics announced the FDA issued an Emergency Use Authorization (EUA)…

On Apr. 10, 2020, Eli Lilly announced it had entered into an agreement with the National Institute of…

On Apr. 10, 2020, a new study has begun recruiting at the NIH in Bethesda, Maryland, to determine…

On Apr. 10, 2020, the Institute for Health Metrics and Evaluation (IHME) released updated COVID-19 predictions for deaths…
On Apr. 9, 2020, Codex DNA announced a newly developed and released suite of products and services designed…

On Apr. 9, 2020, BioSig Technologies announced that an article titled “The IMPDH inhibitor merimepodib suppresses SARS-COV-2 replications”…

On Apr. 9, 2020, Washington University School of Medicine in St. Louis launched a clinical trial for patients…

On Apr. 9, 2020, LabCorp and Ciox Health announced an agreement to collaborate on a comprehensive U.S.-based COVID-19…

On Apr. 9, 2020, BioCryst Pharmaceuticals announced the company has opened enrollment into a randomized, double-blind, placebo-controlled clinical…

On Apr. 9, 2020, Pfizer and BioNTech disclosed additional details of their collaboration to advance candidates from BioNTech’s…

On Apr. 9, 2020, Chembio Diagnostics has been selected for use in a Stony Brook Medicine effort to…

On Apr. 9, 2020, iBio announced the signing of two agreements with the Infectious Disease Research Institute (IDRI)…
On Apr. 9, 2020, CytoDyn announced a patient with severe COVID-19 under the care of a leading medical…
On Apr. 9, 2020, the first participants were enrolled in the trial at Vanderbilt University Medical Center, Nashville,…

On Apr. 9, 2020, CytoDyn announced results of immunological metrics from blood samples of seven severely ill COVID-19…

On Apr. 9, 2020, FUJIFILM announced initiation of a U.S. phase II clinical trial to evaluate the safety…
On Apr. 9, 2020, Laurent Pharmaceuticals announced that a spin off from McGill University planned to test its…

On Apr. 8, 2020, PharmaCyte Biotech announced a license agreement with Hai Kang Life Corp. whereby PharmaCyte received…
On Apr. 8, 2020, Nanomix announced that the company had been awarded $570,000 in funding from Biomedical Advanced…

On Apr. 8, 2020, Vanda Pharmaceuticals and the University of Illinois at Chicago (UIC) announced a research partnership…

On Apr. 8, 2020, Pacific Biosciences announced it is working with commercial, academic and government research teams that…

On Apr. 8, 2020, Bellerophon Therapeutics announced submission of an Investigational New Drug (IND) application to the FDA…

On Apr. 8, 2020, Chugai Pharma announced that it is working to start a phase III clinical trial…

On Apr. 8, 2020, CSL Behring and SAB Biotherapeutics announced a partnership to combat the coronavirus pandemic with…

On Apr. 8, 2020, a team of Texas A&M University researchers led by Michael R. Moreno, in collaboration…

On Apr. 8, 2020, Philips announced that the U.S. Government and Philips agreed to team up to increase…

On Apr. 8, 2020, Abbott announced that the FreeStyle Libre 14 day system, a continuous glucose monitoring (CGM)…