Edwards HemoSphere platform receives expanded use indication from Health Canada in treatment of COVID-19 patients
On Apr. 27, 2020, Edwards Lifesciences announced that Health Canada approved an expanded indication for use of the…
On Apr. 27, 2020, Edwards Lifesciences announced that Health Canada approved an expanded indication for use of the…
On Apr. 25, 2020, the World Health Organization (WHO) there was currently ‘no evidence’ that people who have…

On Apr. 24, 2020, researchers at the University of Oxford announced the start of a large, international study…
On Apr. 24, 2020, the governing Board of the California Institute for Regenerative Medicine (CIRM) awarded $749,999 to…

On Apr. 24, 2020, the W. M. Keck Foundation announced a $4 million gift that will help scientists…

On Apr. 24, 2020, Shelly Miller, Professor of Mechanical and Environmental Engineering, University of Colorado Boulder, reported that…

On Apr. 24, 2020, BioSig Technologies announced that subsidiary ViralClear had submitted an Investigational New Drug (IND) application…

On Apr. 24, 2020, Oregon Health & Science University (OHSU) announced that a research team led by Albert…

On Apr. 24, 2020, the U.S. Food and Drug Administration (FDA) issued a Drug Safety Communication regarding known…

On Apr. 24, 2020, a new study led by researchers at Oxford and Guangdong Centre for Diseases Control…

On Apr. 24, 2020, Mesoblast announced an 83% survival in ventilator-dependent COVID-19 patients (10/12) with moderate/severe acute respiratory…

On Apr. 23, 2020, AstraZeneca and and Saint Lukeメs Mid America Heart Institute initiated a randomised, global Phase…

On Apr. 23, 2020, a study led by researchers at Oxford and Guangdong Centre for Diseases Control and…

On Apr. 23, 2020, the Wellcome Sanger Institute announced that two specific cell types in the nose has…

On Apr. 23, 2020, two significant international studies involving hundreds of scientists, including a human geneticist at the…

On Apr. 23, 2020, Yale researchers began a clinical trial at Yale New Haven Hospital to test the…

On Apr. 23, 2020, BioSig Technologies announced that an article titled, ‘The IMPDH inhibitor merimepodib has similar antiviral…

On Apr. 23, 2020, Emergent BioSolutions announced an agreement whereby Emergent will deploy its contract development and manufacturing…

On Apr. 23, 2020, the Mayo Clinic, Minnesota Department of Health and University of Minnesota announced a breakthrough…

On Apr. 23, 2020, Johnson & Johnson announced a collaboration between the Janssen Pharmaceutical of Johnson & Johnson…

On Apr. 23, 2020, Baxter announced it had received emergency use authorization (EUA) from the FDA for the…

On Apr. 23, 2020, Insmed announced that it will provide funding and clinical drug supply for the STOP-COVID19…

On Apr. 22, 2020, the FDA issued an emergency use authorization (EUA) for the Philips Medizin Systeme Boeblingen…

On Apr. 23, 2020, Caladrius Biosciences announced the FDA has authorized its investigational new drug application for the…
On Apr. 23, 2020, Western New York healthcare and medical research leaders joined forces in an effort to…

On Apr. 23, 2020, a new strategic plan from the National Institute of Allergy and Infectious Diseases (NIAID),…
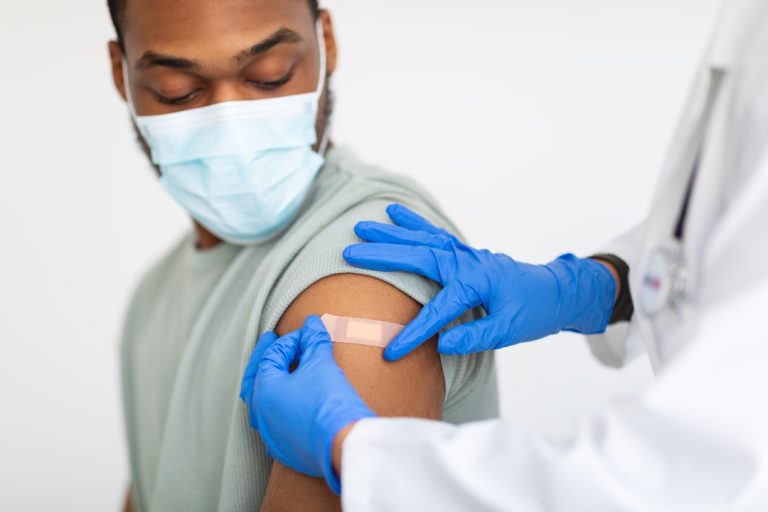
On Apr. 23, 2020, University of Oxford researchers announced they had begun testing a COVID-19 vaccine in human…

On Apr. 23, 2020, researchers from the University of Oxford are supporting a new government study to track…

On Apr. 22, 2020, the Biomedical Advanced Research and Development Authority (BARDA) and Tangen Biosciences announced teaming up…

On Apr. 20, 2020, SNBL announced their intention to participate in the development of a prophylactic DNA vaccine…