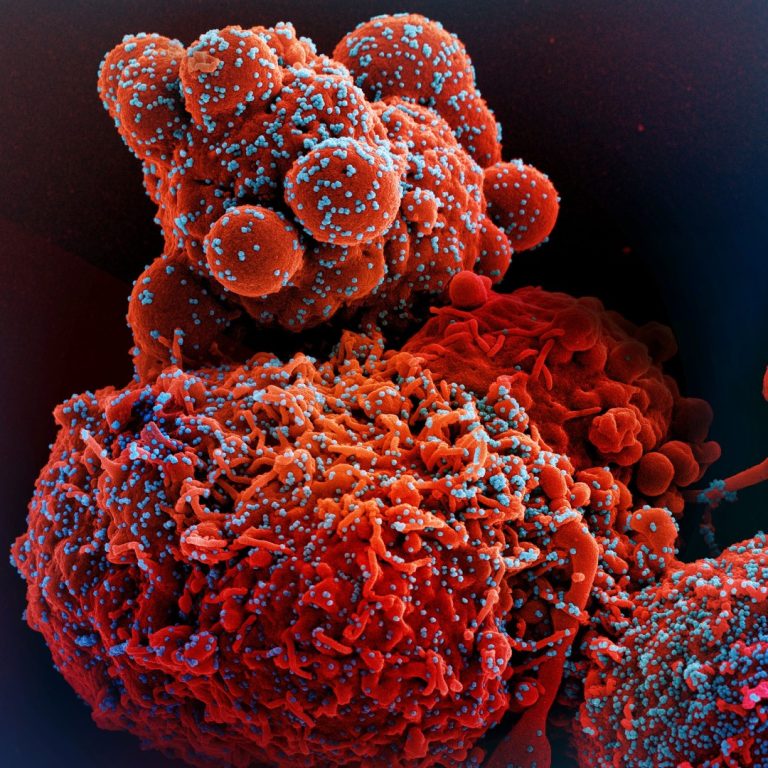
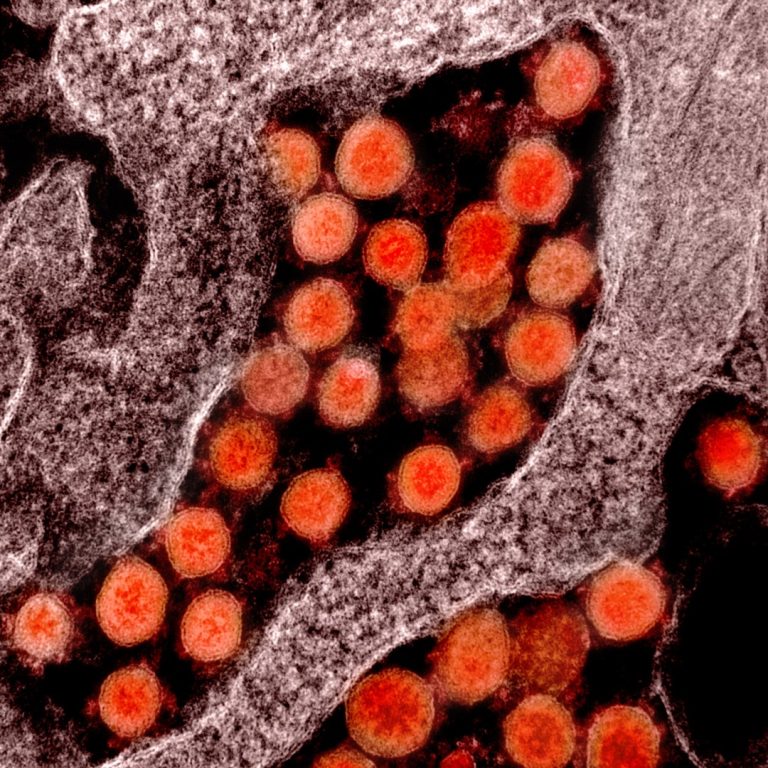
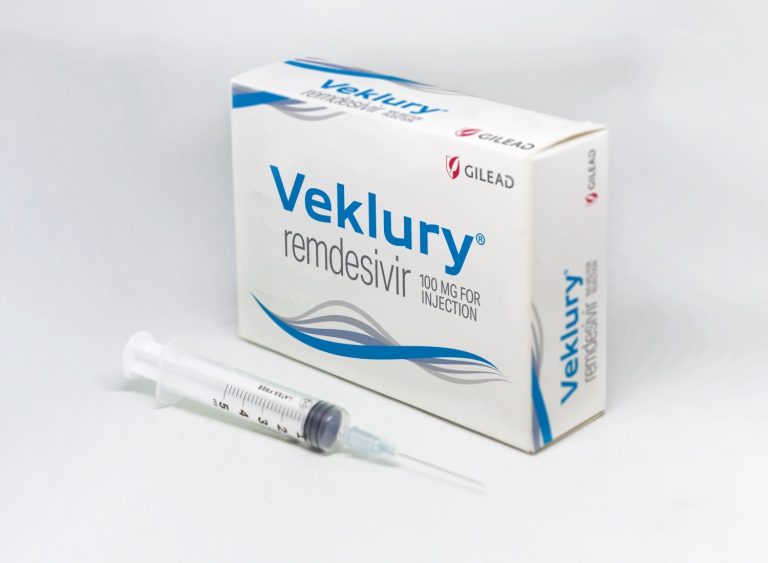
Todos Medical entered Into exclusive distribution agreement with Gnomegen and received FDA Emergency Use Authorization for COVID-19 qPCR test kits
On May 11, 2020, Todos Medical announced an exclusive distribution agreement with Gnomegen for the distribution of its…