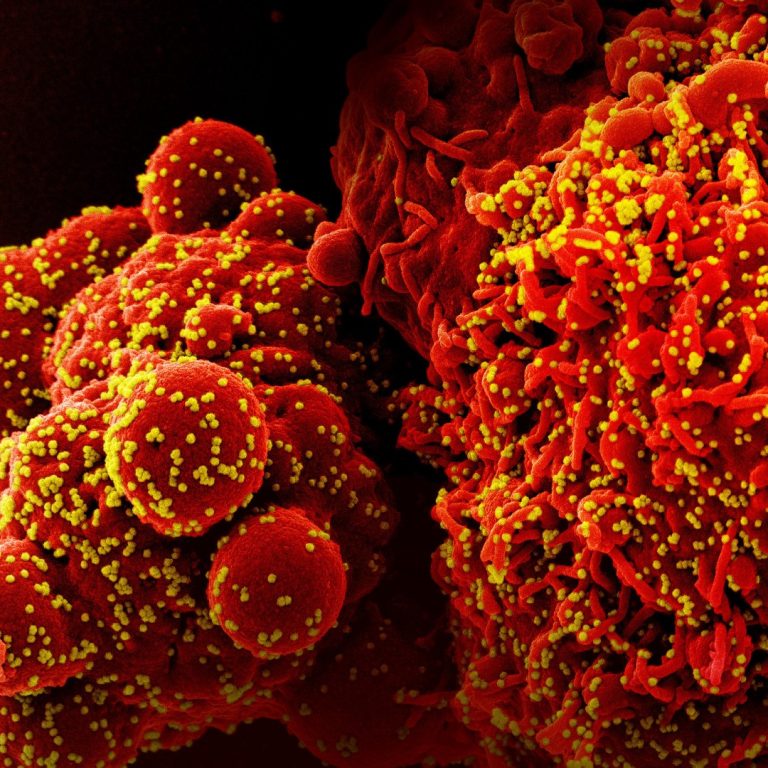
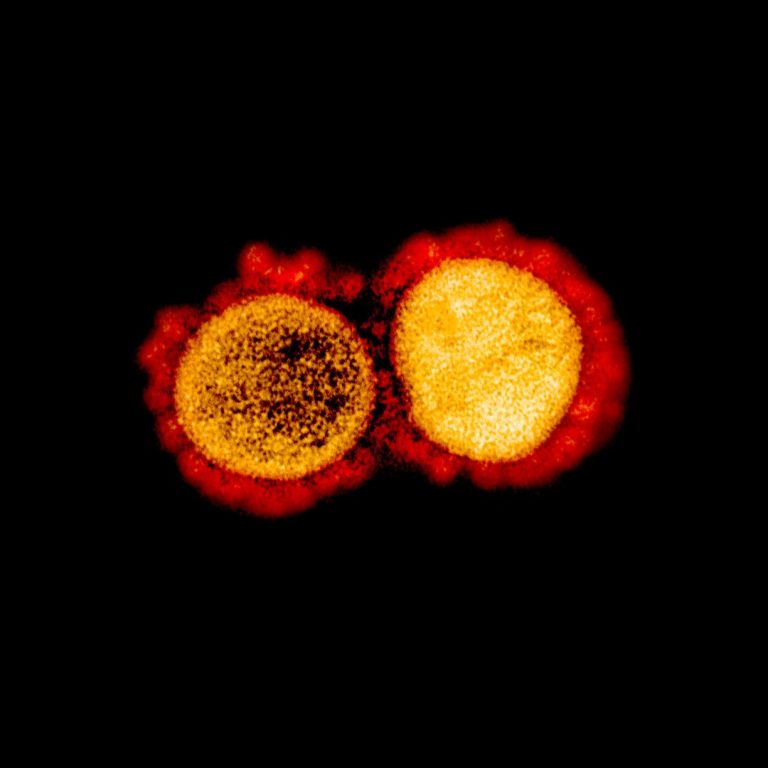
NanoViricides’s antiviral drug candidate for treatment of COVID-19 infections well tolerated in animal safety studies
On Feb. 8, 2021, NanoViricides announced that its broad-spectrum anti-coronavirus drug candidate for the treatment of COVID-19 infections…